The dipole moment of  is 1.5 D. The dipole moment of
is 1.5 D. The dipole moment of 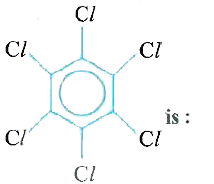 is:
is:
A
0 D
B
1.5 D
C
2.86 D
D
2.25 D
Video Solution
Text Solution
The correct Answer is:A
|
Answer
Step by step video, text & image solution for The dipole moment of is 1.5 D. The dipole moment of is: by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
Dipole moment of
 is 1.5D. The dipole moment of
is 1.5D. The dipole moment of
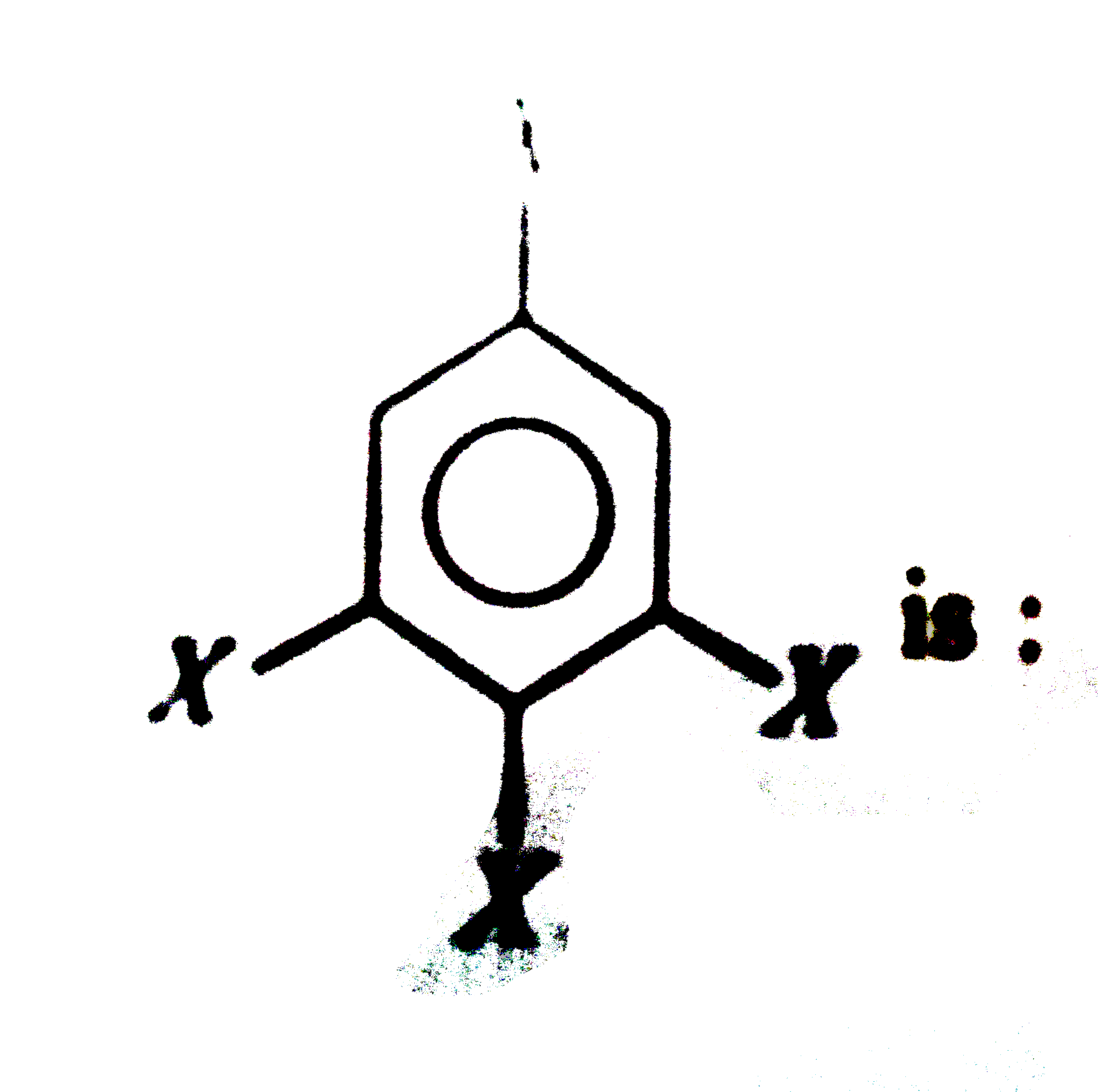 A1DB1.5DC2.25DD3D
A1DB1.5DC2.25DD3DDipole momentof
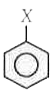 is 1.5 D. The dipole moment of
is 1.5 D. The dipole moment of
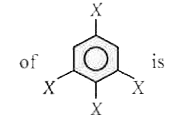 is A1.5 DB2.25 DC1DD3 D
is A1.5 DB2.25 DC1DD3 DDipole moment of
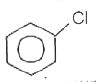 is 1.1 D hence dipole moment of given compound will be
is 1.1 D hence dipole moment of given compound will be
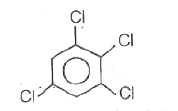 A1.1 DB4.4 DC3.3 DD2.56 D
A1.1 DB4.4 DC3.3 DD2.56 D
The dipole moment is minimum in
View SolutionAn electric dipole is kept on the axis of a uniformly charged ring at distance d from the centre of the ring. The direction of the dipole moment is along the axis. The dipole moment is p, charge of the ring is Q & radius of the ring is R. The force on the dipole is
View SolutionThe dipole moment of chlorobenzene is 1.73 D . The dipole moment of p-dichlorobenzene is expected to be
View SolutionElectric dipole moment is
View SolutionElectric dipole moment is
View Solution

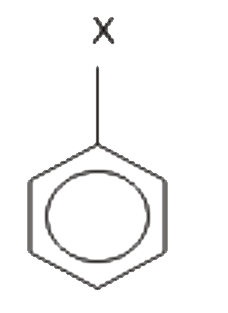 is 1.5 D.
is 1.5 D. 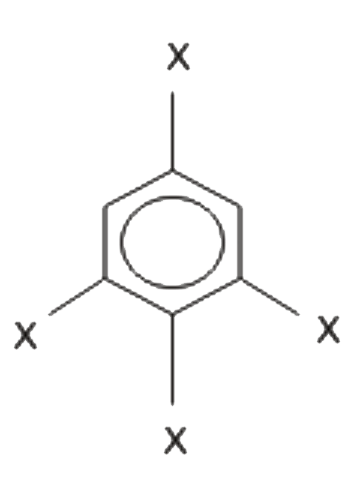 in Debye
in Debye is 1.5D what will be the dipole moment of
is 1.5D what will be the dipole moment of 