Oxidation number if iodine in IO−3,IO−4,KIandI2 respectively are
A
−1,−1,0+1
B
+3,+5,+7,0
C
+5,+7,−1,0
D
−1,−5,−1,0
Video Solution
Text Solution
The correct Answer is:C
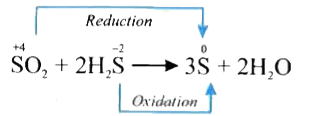
H2S is oxidised in this reaction.
|
Similar Questions
IO−3,IO−4,KI और I2 में आयोडीन की ऑक्सीकरण संख्या क्रमश: है
View SolutionHIO4,H3IO5 और H5IO6 में आयोडिन की ऑक्सीकरण अवस्था क्रमश: होगी
View SolutionOxidation number of iodine in IO_(3)^(-), IO_(4)^(-), KI and I_(2) respectively are:
View SolutionOxidation number of iodine in IO_(3)^(-),IO_(4)^(-),KIandI_(2) respectively are ____
View SolutionH_(4)IO_(6)^(-) The oxidation state of iodine in
View SolutionOxidation number if iodine in IO−3,IO−4,KIandI2 respectively are
View SolutionIO−3,IO−4,KI तथा I2 में आयोडीन के ऑक्सीकरण संख्या क्रमशः है-
View SolutionThe oxidation states of iodine in HIO4,H3IO5 and H5IO6 are ______________ respectively.
View Solution
