Complete the following Reaction
CH3−CH=CH2+HBr
CCl3−CH=CH2+HBr
To complete the reactions given in the question, we will follow the Markovnikov's rule for the addition of HBr to alkenes. Let's break down the steps for each reaction.
Reaction 1: CH₃-CH=CH₂ + HBr
1. Identify the Alkene: The compound CH₃-CH=CH₂ is an alkene with a double bond between the second and third carbon atoms.
2. Apply Markovnikov's Rule: According to Markovnikov's rule, when HBr adds to an alkene, the hydrogen atom (H) will attach to the carbon with the greater number of hydrogen atoms already attached, while the bromine atom (Br) will attach to the carbon with fewer hydrogen atoms.
3. Determine the Products:
- The double bond is between the second and third carbon atoms. The second carbon (CH) has one hydrogen, and the third carbon (C=CH₂) has two hydrogens.
- Therefore, H will add to the second carbon (CH), and Br will add to the third carbon (C=CH₂).
- The resulting product will be CH₃-CHBr-CH₃.
4. Final Product: The complete reaction is:
CH₃−CH=CH₂+HBr→CH₃−CHBr−CH₃
Reaction 2: CCl₃-CH=CH₂ + HBr
1. Identify the Alkene: The compound CCl₃-CH=CH₂ is also an alkene with a double bond between the second and third carbon atoms.
2. Apply Markovnikov's Rule: Again, we will apply Markovnikov's rule for the addition of HBr. The hydrogen atom will attach to the carbon with more hydrogen atoms, and the bromine will attach to the carbon with fewer hydrogen atoms.
3. Determine the Products:
- The second carbon (CCl₃-CH) has one hydrogen, and the third carbon (C=CH₂) has two hydrogens.
- Thus, H will add to the third carbon (C=CH₂), and Br will add to the second carbon (CCl₃-CH).
- The resulting product will be CCl₃-CH-CH₂Br.
4. Final Product: The complete reaction is:
CCl₃−CH=CH₂+HBr→CCl₃−CH−CH₂Br
Summary of Products
1. For the first reaction:
CH₃−CH=CH₂+HBr→CH₃−CHBr−CH₃
2. For the second reaction:
CCl₃−CH=CH₂+HBr→CCl₃−CH−CH₂Br
2. Apply Markovnikov's Rule: According to Markovnikov's rule, when HBr adds to an alkene, the hydrogen atom (H) will attach to the carbon with the greater number of hydrogen atoms already attached, while the bromine atom (Br) will attach to the carbon with fewer hydrogen atoms.
- The double bond is between the second and third carbon atoms. The second carbon (CH) has one hydrogen, and the third carbon (C=CH₂) has two hydrogens.
- Therefore, H will add to the second carbon (CH), and Br will add to the third carbon (C=CH₂).
- The resulting product will be CH₃-CHBr-CH₃.
CH₃−CH=CH₂+HBr→CH₃−CHBr−CH₃
- The second carbon (CCl₃-CH) has one hydrogen, and the third carbon (C=CH₂) has two hydrogens.
- Thus, H will add to the third carbon (C=CH₂), and Br will add to the second carbon (CCl₃-CH).
- The resulting product will be CCl₃-CH-CH₂Br.
CCl₃−CH=CH₂+HBr→CCl₃−CH−CH₂Br
1. For the first reaction:
CH₃−CH=CH₂+HBr→CH₃−CHBr−CH₃
2. For the second reaction:
CCl₃−CH=CH₂+HBr→CCl₃−CH−CH₂Br
Similar Questions
Knowledge Check
The reaction CH2=CHCH3=HBr→CH3CHBrCH3 is-
ANucleophilic additionBElectrophilic additionCElectrophilic substitutionDfree radical additionWhich reagent would you use for the following reaction ?
CH3CH2CH2CH3→CH3CH2CH2Cl+CH3CH2CHClCH3ACl2/UV lightBNaCl+H2SO4CCl2 gas in darkDCl2 gas in the presence of iron in darkThe major product of the following reaction is:
CH3−CH=CH2+HBr(C6H5CO)2O2 peroxide−−−−−−−−−−−−−−−→ACH3−CH2−CH2−BrBCH2CH(Br)−CH3CBrCH2−CH=CH2D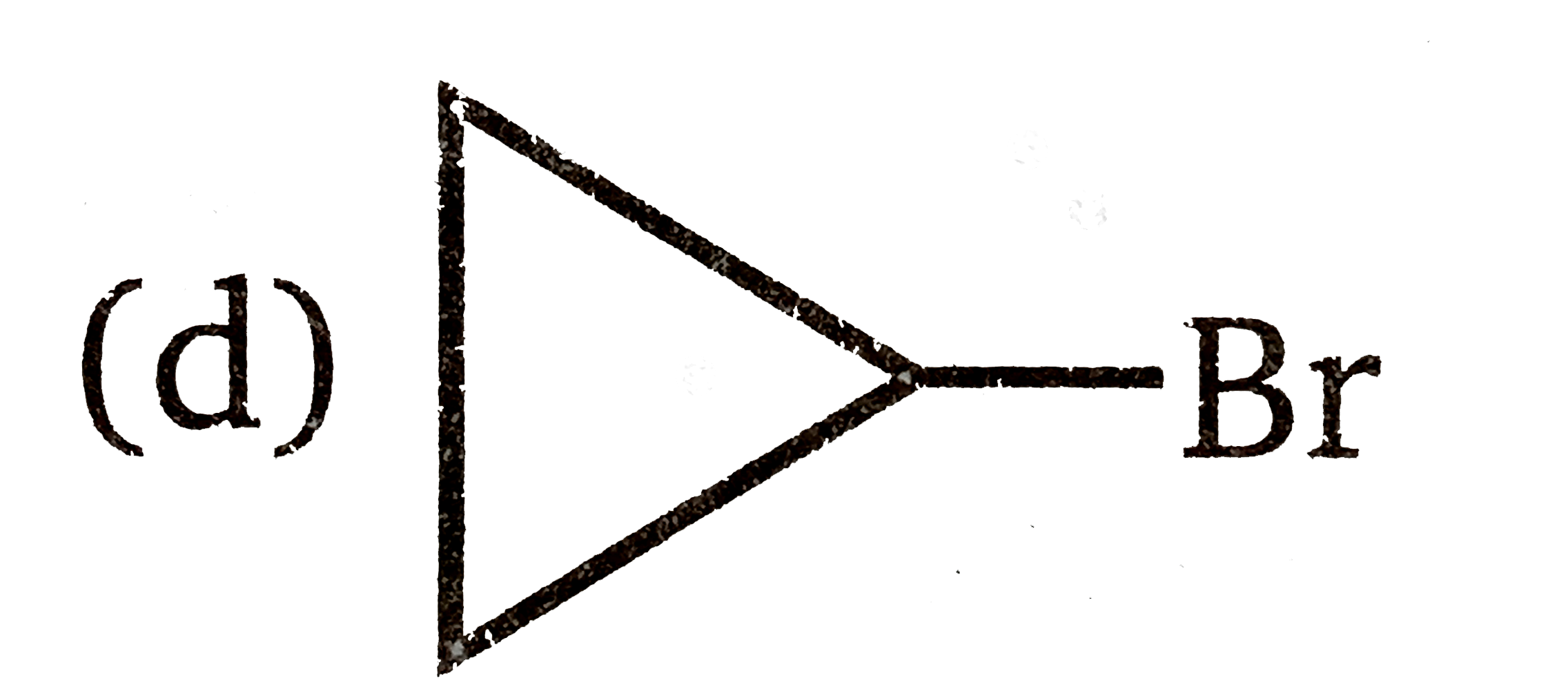
Complete the following reactions:
CH3CH2CH2OHSOCl2−−−→?alc.KOH−−−−−→?HBr−−→?aq.KOH−−−−→
View SolutionComplete the following chemicals equation
CH3CH2CH=CH2+HBr(peroxide)−−−−−−→
View SolutionComplete the following reaction:
CH3−CH=CH2HBr−−−−→PeroxideXNaI−−−−→AcetoneY.
View SolutionWrite the structures of the major products in the following reactions :
(i)CH3CH=C(CH3)2+HBr→(ii)C6H5ONa+C2H5Cl→
View SolutionWrite the structures of the product of the following reactions:
(i) CH3CH=C(CH3)2+HBr→
(ii). C6H5ONa+C2H5Cl→
View Solution

