Which of the following can produce a racemic mixture on monobromination?
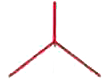

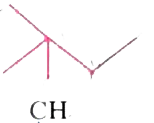
CH3−CH3
The correct Answer is:C
To determine which compound can produce a racemic mixture upon monobromination, we need to analyze each option based on the presence of different types of hydrogen atoms. A racemic mixture is formed when a reaction produces two enantiomers in equal amounts, which typically occurs when there is a chiral center created during the reaction.
1. Understanding Monobromination:
- Monobromination refers to the substitution of one hydrogen atom with a bromine atom in a hydrocarbon. This process can lead to the formation of different products depending on the structure of the hydrocarbon.
2. Identifying Chiral Centers:
- A chiral center is a carbon atom that is bonded to four different substituents. The formation of a racemic mixture typically occurs when a chiral center is generated during the reaction.
3. Analyzing Each Compound:
- Option 1: If the compound has only one type of hydrogen, monobromination will yield a single product, which will not be a racemic mixture.
- Option 2: Similar to option 1, if all hydrogens are equivalent, only one product will be formed, leading to no racemic mixture.
- Option 3: If the compound has different types of hydrogens (for example, a secondary carbon and a tertiary carbon), monobromination can lead to the formation of two different products, which can be enantiomers. This would result in a racemic mixture.
- Option 4: If the compound does not create a chiral center upon bromination, it will not produce a racemic mixture.
4. Conclusion:
- After analyzing the options, the compound in Option 3 is the one that can produce a racemic mixture upon monobromination because it has different types of hydrogen atoms that can lead to the formation of enantiomers.
Final Answer:
Option 3 can produce a racemic mixture on monobromination.
- Monobromination refers to the substitution of one hydrogen atom with a bromine atom in a hydrocarbon. This process can lead to the formation of different products depending on the structure of the hydrocarbon.
- A chiral center is a carbon atom that is bonded to four different substituents. The formation of a racemic mixture typically occurs when a chiral center is generated during the reaction.
- Option 1: If the compound has only one type of hydrogen, monobromination will yield a single product, which will not be a racemic mixture.
- Option 2: Similar to option 1, if all hydrogens are equivalent, only one product will be formed, leading to no racemic mixture.
- Option 3: If the compound has different types of hydrogens (for example, a secondary carbon and a tertiary carbon), monobromination can lead to the formation of two different products, which can be enantiomers. This would result in a racemic mixture.
- Option 4: If the compound does not create a chiral center upon bromination, it will not produce a racemic mixture.
- After analyzing the options, the compound in Option 3 is the one that can produce a racemic mixture upon monobromination because it has different types of hydrogen atoms that can lead to the formation of enantiomers.
Option 3 can produce a racemic mixture on monobromination.
Similar Questions
Knowledge Check
Which of the following can produce silicon.
AReduction of SiO2 by carbonBReaduction of SiO2 by silicon carbideCThermal decomposition of SiH4DThermal decomposition of SiCWhich of the following can produce a regular reflection?
AClothBNewspaperCTowelDMetal plateWhich one of the following products would produce a racemic mixture on monochlorination ?
I.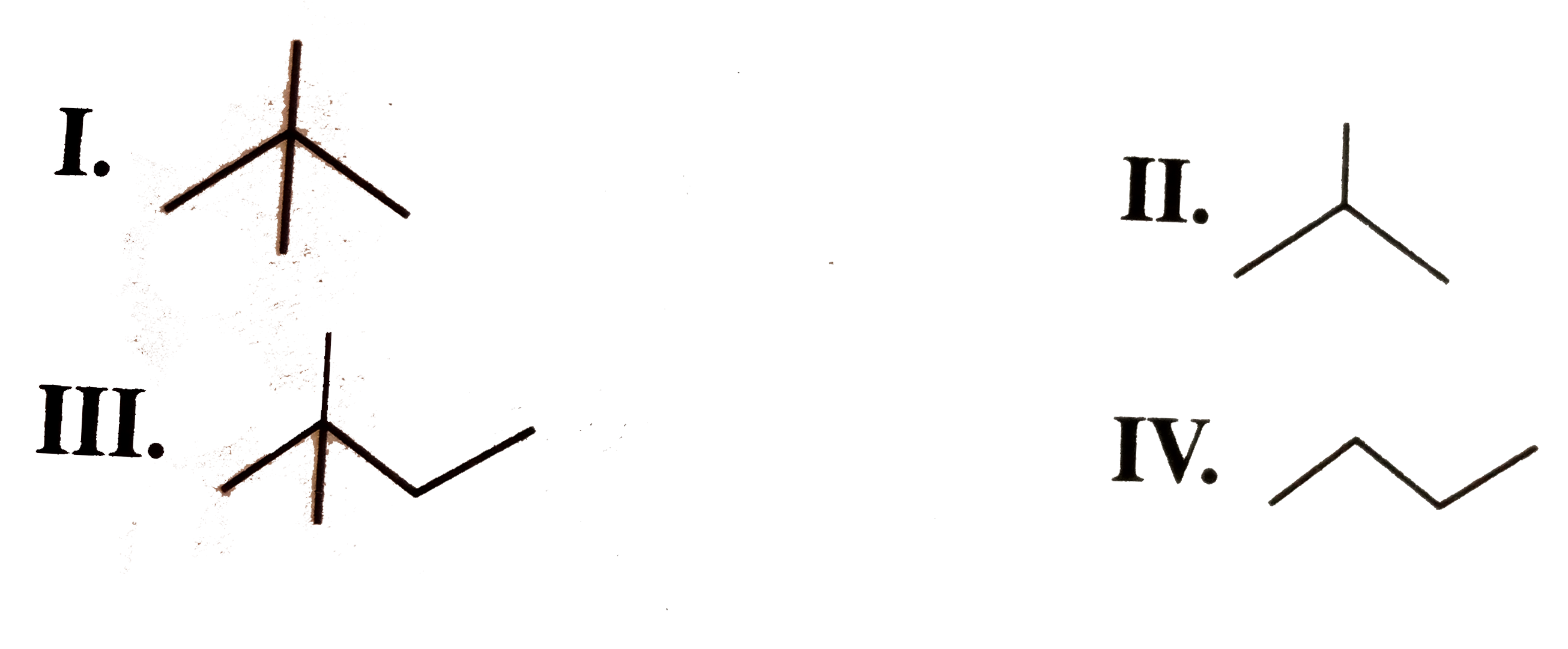 AI, IIBII, IIICII, IVDIII, IV
AI, IIBII, IIICII, IVDIII, IV
Which of the following can produce maximum induced emf?
View SolutionWhich of the following is a racemic mixture?
View SolutionWhich of the following reaction will give racemic mixture ?
View SolutionWhich of the following can produce hydrogen from water
View SolutionWhich of the following can produce hydrogen from water
View Solution

