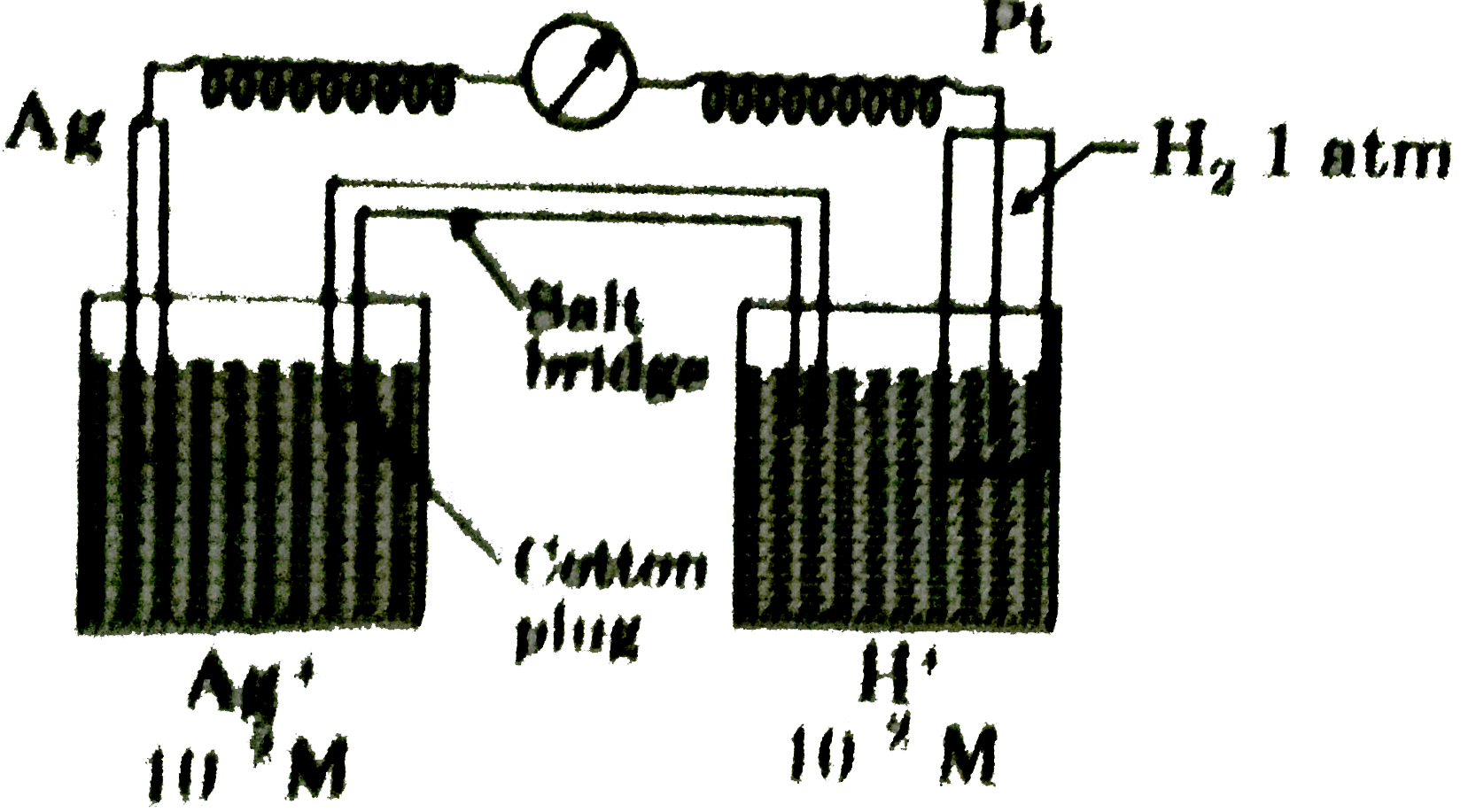
Calculate the emf of given cell
(Ag|AgNO3||AgNO3|Ag,,EAg+/Ag=0.80V),(0.001M,0.1M)
Text Solution
The given cell is concentrated cell. Hence, E∘cell=0
Ecell=E∘cell−0.0591nlog[(product)](Reactant)
Ecell=0−0.05911log10−310−1=0.118 volt
|
Similar Questions
Knowledge Check
Calculate emf of given cell at 25∘C:
[Given: E∘Ag+/Ag)=0.80V]A+0.80VB0.12 VC−0.178VD1.04 VCalculate the emf of the following concentration cell at 25∘C:
Ag(s)|AgNO3(0.01M)||AgNO3(0.05M)|Ag(s)A−0.414VB0.828VC0.414VD0.0414VCalculate the emf of the following concentration cell at 25∘C:
Ag(s)|AgNO3(0.01M)||AgNO3(0.05M)|Ag(s)A0.828VB0.0413VC−0.0413 VD−0.828 V
Calculate the emf of the cell.
Mg(s)∣∣Mg2+(0.2M)∣∣∣∣Ag+(1×10−3)∣∣Ag
E∘Ag+/Ag=+0.8 volt, E∘Mg2+/Mg=−2.37 volt
What will be the effect on emf If concentration of Mg2+ ion is decreased to 0.1M ?
View SolutionCalculate the emf of the cell.
Mg(s)∣∣Mg2+(0.2M)∣∣∣∣Ag+(1×10−3)∣∣Ag
E∘Ag+/Ag=+0.8 volt, E∘Mg2+/Mg=−2.37 volt
What will be the effect on emf If concentration of Mg2+ ion is decreased to 0.1M ?
View SolutionCalculate the emf of the following cell at 25∘C
Ag(s)∣∣AgNO3(0.01 mol Kg−1)∣∣AgNO3(0.05 mole Kg−1)∣Ag(s)
View SolutionCalculate the emf of the following concentration cell at 25∘C:
Ag(s)|AgNO3(0.01M)||AgNO3(0.05M)|Ag(s)
View SolutionThe standard oxidation ptoetial of zinic is 0.76 V and of silver is -0.80 V calculate the emf of the cell
Zn∣∣Zn(NO3)2(0.25M)∣∣|AgNO3(0.1)M|Agat25∘C
View Solution

