The following is an example of Sandmeyer reaction
C6H5N+2Cl−CuCl/HCl−−−−−−→C6H5Cl
C6H5N+2Cl−H2O/Δ−−−−→C6H5OH
C6H5N+2Cl−HBF4−−−→C6H5F
C6H5N+2Cl−KI/warm−−−−−→C6H5I
The correct Answer is:A
To determine which of the given reactions is an example of the Sandmeyer reaction, we need to understand the key components and conditions of the Sandmeyer reaction.
1. Identify the Sandmeyer Reaction:
- The Sandmeyer reaction involves the conversion of an aryl diazonium salt into a haloarene (chlorobenzene or bromobenzene) using cuprous halides (CuX) in the presence of hydrogen halide (HX).
2. Analyze the Given Options:
- Option A: Benzene diazonium chloride reacts with CuCl and HCl to form chlorobenzene.
- Option B: Benzene diazonium chloride reacts with water and heat to form phenol.
- Option C: Benzene diazonium chloride reacts with HBF4 to form fluorobenzene.
- Option D: Benzene diazonium chloride reacts with potassium iodide to form iodobenzene.
3. Evaluate Each Option:
- Option A: This matches the Sandmeyer reaction criteria (CuCl + HCl). Therefore, this is a Sandmeyer reaction.
- Option B: This involves hydrolysis of the diazonium salt to form phenol, which is not a Sandmeyer reaction.
- Option C: This uses HBF4 to produce fluorobenzene, which is not part of the Sandmeyer reaction (only Cl and Br are formed).
- Option D: This uses potassium iodide to produce iodobenzene, which again does not fit the Sandmeyer criteria.
4. Conclusion:
- The only option that represents a Sandmeyer reaction is Option A, where benzene diazonium chloride reacts with CuCl and HCl to yield chlorobenzene.
Final Answer:
Option A is the example of the Sandmeyer reaction.
- The Sandmeyer reaction involves the conversion of an aryl diazonium salt into a haloarene (chlorobenzene or bromobenzene) using cuprous halides (CuX) in the presence of hydrogen halide (HX).
- Option A: Benzene diazonium chloride reacts with CuCl and HCl to form chlorobenzene.
- Option B: Benzene diazonium chloride reacts with water and heat to form phenol.
- Option C: Benzene diazonium chloride reacts with HBF4 to form fluorobenzene.
- Option D: Benzene diazonium chloride reacts with potassium iodide to form iodobenzene.
- Option A: This matches the Sandmeyer reaction criteria (CuCl + HCl). Therefore, this is a Sandmeyer reaction.
- Option B: This involves hydrolysis of the diazonium salt to form phenol, which is not a Sandmeyer reaction.
- Option C: This uses HBF4 to produce fluorobenzene, which is not part of the Sandmeyer reaction (only Cl and Br are formed).
- Option D: This uses potassium iodide to produce iodobenzene, which again does not fit the Sandmeyer criteria.
- The only option that represents a Sandmeyer reaction is Option A, where benzene diazonium chloride reacts with CuCl and HCl to yield chlorobenzene.
Option A is the example of the Sandmeyer reaction.
Topper's Solved these Questions
- HALOALKANE AND HALOARENESBook:NARAYNAChapter:HALOALKANE AND HALOARENESExercise:EXERCISE - II (C.W)
Explore 28 Videos
- HALOALKANE AND HALOARENESBook:NARAYNAChapter:HALOALKANE AND HALOARENESExercise:EXERCISE - II (H.W)
Explore 28 Videos
- HALOALKANE AND HALOARENESBook:NARAYNAChapter:HALOALKANE AND HALOARENESExercise:EXERCISE - I (C.W)
Explore 30 Videos
- F-BLOCK ELEMENTSBook:NARAYNAChapter:F-BLOCK ELEMENTSExercise:CHECK YOUR GRASP
Explore 2 Videos
- HALOGEN COMPOUNDSBook:NARAYNAChapter:HALOGEN COMPOUNDSExercise:Passage 13
Explore 1 Video
Similar Questions
Knowledge Check
Which one of the following are examples of Sandmeyer reaction?
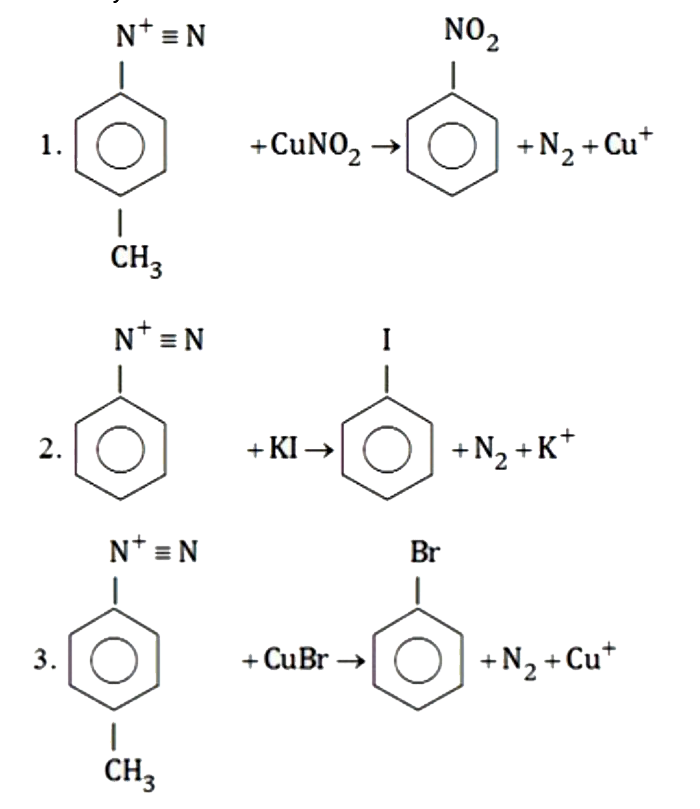
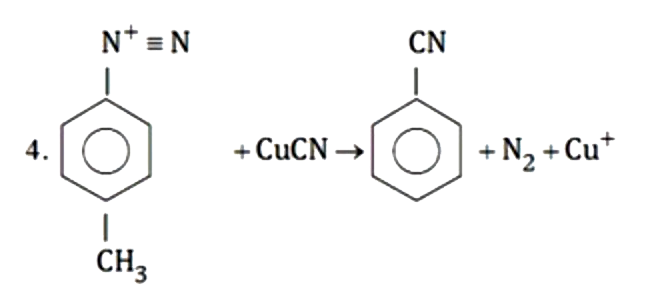 A1, 2, 4B1, 2, 3C1, 3, 4D1, 2, 3, 4
A1, 2, 4B1, 2, 3C1, 3, 4D1, 2, 3, 4Which of the following is not an example of Sandmeyer’s reaction ?
AC6H5+N2−ClCuCl−−→C6H5ClBC6H5+N2−ClCuBr−−→C6H5BrCC6H5+N2−ClCuCN/KCN−−−−−−−−→C6H5CNDC6H5+N2−ClKI/warm−−−−→C6H5IWhich of the following are examples of Sandmeyer's reaction?
AC6H5N+2ClCuCl−−→C6H5ClBC6H5N+2ClCu2(CN)2−−−−−−→C6H5CNCC6H5N+2ClNaNO2/Cu/Δ−−−−−−−−−→C6H5NO2DC6H5N+2ClKl/Δ−−−→C6H5I
Which of the following is an example of endothermic reaction
View SolutionWhich of the following is an example of Stephen reaction?
View SolutionWhich of the following is an example of redox reaction ?
View SolutionWhich of the following is an example of redox reaction ?
View SolutionWhich of the following is an example of elimination reaction ?
View Solution
