Addition of to the aqueous hydrolysis of benzyl chloride dose not increase the rate of the hydrolysis but change only the composition of the final products. This indicates that the reaction is proceeding through
SN1 mechanisms
SN1 mechanisms
SE1 mechanisms
SE2 mechanisms
The correct Answer is:A
Rate is depenending only on one reactant . iodination of benzene is a reversible reaction
Similar Questions
If doubling the concentration of a reactant X in a reaction X+Y→ Products, increases the rate times and tripling its concentration increases the rate nine times, this indicates that the rate of reaction is proportional to the ………….of the concentration of.............and thus rate is given by........... .
View SolutionFor the reaction 2A+B→Products it is found that doubling the concentration of both reactants increases the rate by a factor of 8. But doubling the concentration of "B" alone only doubles the rate.What is the order of the reaction w.r.t to A?
View Solution
Knowledge Check
In alkaline hydrolysis of a tertiary halide by aqueous alkali, if concentration of alkali is doubled, then the reaction rate
Awill be boubledBwill be halvedCwill remain constantDCannot say anythingFor the reaction A+B products, it is observed that:
(1) on doubling the initial concentration of A only, the rate of reaction is also doubled and
(2) on doubling te initial concentration of both A and B, there is a charge by a factor of 8 in the rate of the reaction.
The rate of this reaction is given byARate = K[A][B]2BRate = K[A]2[B]2CRate = k [A][B]DRate = K[A]2[B]Consider a solution of CH3COONH4 which is a salt weak acid and weak base.
The equilibrium involved in the solutions are :
CH3COO−+H2O⇔CH3COOH+OH− ........(i)
NH+4+H2⇔NH4OH+H+ ........(ii)
H++OH−⇔H2O ........(iii)
If we add these reactions, then the net reaction is :
CH3COO−+H+2+H2O⇔CH3COOH+NH4OH ........(iv)
Both CH3COO−and NH+4 get hydrolysed independently and their hydrolysis depends on :
(a) their initial concentration
(b) The value of Kh which is KwKa for CH3COO− and KwKb for NH+4.
Since both of the ions were produced form the salt, their initial concertration are same. Therefore, unless and until the value of KwKa or Ka and Kb is same, the degree of hydrogen of ions can't be same.
To explain why we assume that degree of hydrolysis of cation and anion is same, we needed to now look at the third reaction i.e.,combination of H+ and OH− ions. It is obvious that this reaction happens only because one reaction produced H+ ion and the other prodcued OH− ions. We can also note that this reaction causes both the hydrolysis reaction to occur more since their product ions are being consumed. Keep this in mind that the equilibrium which has smaller value of the equilibrium constant is affected more by the common ion effect. For the same reason if for any reson a reaction is made to occur to a greater extent by the consumption of any of the prodcut ion, the reaction with the smaller value of equilibrium constant tends to get affected more.
Therefore, we conclude that firstly the hydroylsis of both the ions occurs more in the presence of each other (due to consumption of the product ions) than in each other's absence. Secondly,the hydroylsis of the ion which occurs to a lesser extent (due to smaller value of Kh)is affected more than the one whole Kh is greater. Hence we can see that the degree of hydroylsis of both the ions would be close to each other when they are getting hyderolysed in the presence of each other.
In the hydrolysis of salt weak acid and weak base :Adegree of hydrolysis of cation and anion is different.Bdegree of hydrolysis of cation and anion is same.Cdegree of hydrolysis of cation and anion is different but they can never be assumed same.Ddegree of hydrolysis of cation and anion is different but they are very close to each other when they are getting hydrolysed in the presence of each other.
The enthalpy in the process HCl+nH2O→HCl in n moles of H2O Where n is the number of moles of water, is called the integral heat of solution.When n is large enough that continued addition of water does not increase the heat of solution, one simply writes
HCl+aq→HCl(aq)
The enthalpy for this process is the limiting value for the integral heat of solution.The enthalpy for the process
HCl in n moles of H2O→ HCl in (n+m) moles of H2O
is called the integral heat of dilution.These quantities are indicated in figure.Another quantity of interest is the differential heat of solution, defined as the slope of the enthalpy curve. The heats of solution depend on the composition of the solution as shown in the figure.
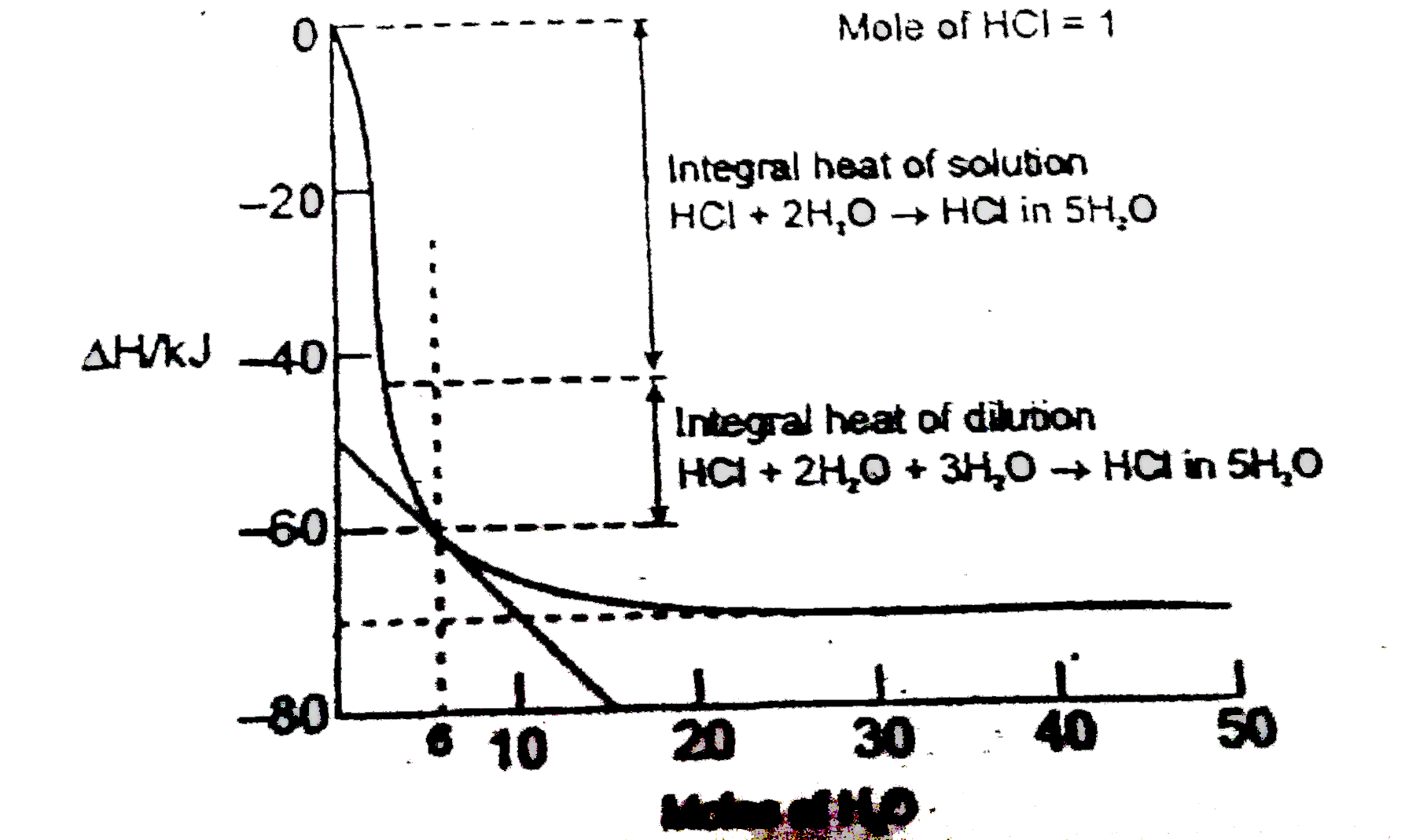
What is the approximate enthalpy change for the reaction HCl+aq→HCl(aq)
View SolutionConsider a solution of CH3COONH4 which is a salt weak acid and weak base.
The equilibrium involved in the solutions are :
CH3COO−+H2O⇔CH3COOH+OH− ........(i)
NH+4+H2⇔NH4OH+H+ ........(ii)
H++OH−⇔H2O ........(iii)
If we add these reactions, then the net reaction is :
CH3COO−+H+2+H2O⇔CH3COOH+NH4OH ........(iv)
Both CH3COO−and NH+4 get hydrolysed independently and their hydrolysis depends on :
(a) their initial concentration
(b) The value of Kh which is KwKa for CH3COO− and KwKb for NH+4.
Since both of the ions were produced form the salt, their initial concertration are same. Therefore, unless and until the value of KwKa or Ka and Kb is same, the degree of hydrogen of ions can't be same.
To explain why we assume that degree of hydrolysis of cation and anion is same, we needed to now look at the third reaction i.e.,combination of H+ and OH− ions. It is obvious that this reaction happens only because one reaction produced H+ ion and the other prodcued OH− ions. We can also note that this reaction causes both the hydrolysis reaction to occur more since their product ions are being consumed. Keep this in mind that the equilibrium which has smaller value of the equilibrium constant is affected more by the common ion effect. For the same reason if for any reson a reaction is made to occur to a greater extent by the consumption of any of the prodcut ion, the reaction with the smaller value of equilibrium constant tends to get affected more.
Therefore, we conclude that firstly the hydroylsis of both the ions occurs more in the presence of each other (due to consumption of the product ions) than in each other's absence. Secondly,the hydroylsis of the ion which occurs to a lesser extent (due to smaller value of Kh)is affected more than the one whole Kh is greater. Hence we can see that the degree of hydroylsis of both the ions would be close to each other when they are getting hyderolysed in the presence of each other.
For 0.1 M CH3COONH4 salt solution given, Ka(CH3COOH)=Kb(NH4OH)=2×10−5.
In the case : degree of hydrolysis of cation and anion are :
View SolutionConsider a solution of CH3COONH4 which is a salt weak acid and weak base.
The equilibrium involved in the solutions are :
CH3COO−+H2O⇔CH3COOH+OH− ........(i)
NH+4+H2⇔NH4OH+H+ ........(ii)
H++OH−⇔H2O ........(iii)
If we add these reactions, then the net reaction is :
CH3COO−+H+2+H2O⇔CH3COOH+NH4OH ........(iv)
Both CH3COO−and NH+4 get hydrolysed independently and their hydrolysis depends on :
(a) their initial concentration
(b) The value of Kh which is KwKa for CH3COO− and KwKb for NH+4.
Since both of the ions were produced form the salt, their initial concertration are same. Therefore, unless and until the value of KwKa or Ka and Kb is same, the degree of hydrogen of ions can't be same.
To explain why we assume that degree of hydrolysis of cation and anion is same, we needed to now look at the third reaction i.e.,combination of H+ and OH− ions. It is obvious that this reaction happens only because one reaction produced H+ ion and the other prodcued OH− ions. We can also note that this reaction causes both the hydrolysis reaction to occur more since their product ions are being consumed. Keep this in mind that the equilibrium which has smaller value of the equilibrium constant is affected more by the common ion effect. For the same reason if for any reson a reaction is made to occur to a greater extent by the consumption of any of the prodcut ion, the reaction with the smaller value of equilibrium constant tends to get affected more.
Therefore, we conclude that firstly the hydroylsis of both the ions occurs more in the presence of each other (due to consumption of the product ions) than in each other's absence. Secondly,the hydroylsis of the ion which occurs to a lesser extent (due to smaller value of Kh)is affected more than the one whole Kh is greater. Hence we can see that the degree of hydroylsis of both the ions would be close to each other when they are getting hyderolysed in the presence of each other.
In a solution of NaHCO3, the amphroximately same undergo ionization to form H+ ion and hydrolysis to form OH− ion.
HCO−3+H2Oionization⇐===⇒CO2−3+H2O
HCO−3+H2Ohydrolysis⇐===⇒H3CO2+OH−
To calculate ph, suitable approximation is :
View SolutionFor the reaction A+B products, it is observed that:
(1) on doubling the initial concentration of A only, the rate of reaction is also doubled and
(2) on doubling te initial concentration of both A and B, there is a charge by a factor of 8 in the rate of the reaction.
The rate of this reaction is given by
View SolutionThe expression for the reaction quotient , Q, is similar to that for equilibrim constant , Q, is similar to that for equilibrium constant K. The value of Q for the given composition of a reaction mixture helps us to know whether the reaction will move forward or backward or remain in equilibrium . It also helps to predict the effect of pressure on the direction of the gaseous reaction .In certain reactions, addition of inert gas also favours either the formation of reactants or products . The value of equilibrium constant of a reaction changes with change of temperature and the change is given by van't Hoff equation , d In Kp/dT=ΔH∘/RT2 where enthaply change, ΔH∘ , is taken as constant in the small temperature range.
Which of the following will be correct ?
View Solution

