Which of the following reagents would not be a good choice for reducing an aryl nitro compound to an amine?
A
H2(excess)Pt
B
LiAH4 in ether
C
Fe and HCl
D
Sn and HCl
Video Solution
Text Solution
The correct Answer is:B
LiAIH4/ether reduces aryl nitro compounds to a azo compounds
2C6H5NO2nitrobenzeneLiAIH4/ether−−−−−−−−→C6H5=N−C6H5diazobenzene
|
Topper's Solved these Questions
- ORGANIC COMPOUNDS CONTAINING NITROGENBook:NARAYNAChapter:ORGANIC COMPOUNDS CONTAINING NITROGENExercise:EXERCISE-4
Explore 30 Videos
- ORGANIC COMPOUNDS CONTAINING NITROGENBook:NARAYNAChapter:ORGANIC COMPOUNDS CONTAINING NITROGENExercise:EXERCISE-2 H.W.
Explore 24 Videos
- NUCLEAR CHEMISTRYBook:NARAYNAChapter:NUCLEAR CHEMISTRYExercise:INTEGER
Explore 28 Videos
- PERIODIC TABLE Book:NARAYNAChapter:PERIODIC TABLE Exercise:All Questions
Explore 573 Videos
Similar Questions
Knowledge Check
Which of the following reagents would not be a good choice for reducing an aryl nitro compound to an amine?
AH2 (excess)/PtBLiAlh4 in etherCFe and HClDSn and HClWhich of the following reduces Tollen's reagent?
A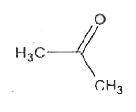 B
B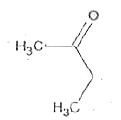 C
C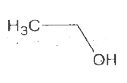 D
D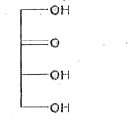
Which of the following reduces Tollen's reagent ?
ACane sugarBStarchCGlucoseDAll of these
Which of the following reagents is not able to reduce an ester into an alcohol?
View SolutionWhich of the following reagents will reduce phenol to benzene?
View SolutionWhich of the following compounds is not an amine ?
View SolutionWhich of the following in not nitro compounds
View SolutionWhich of the following compounds is an amine ?
View Solution
