Calculate the empirical and molecular formula of a compound containing 76.6% carbon, 6.38 % hydrogen and rest oxygen its vapour density is 47Solution in Tamil
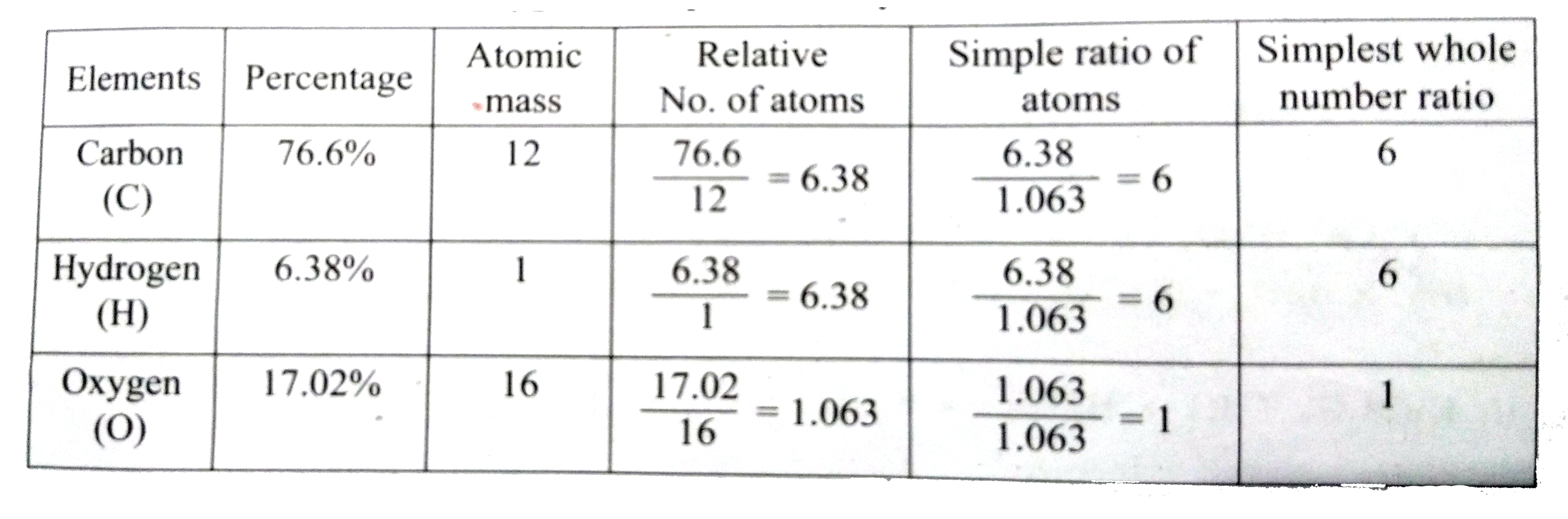
Emperical formula=C6(H6O
Vapour density=47
∴Molecular mass= 2× vapour density
=2×47=94
Molecular formula= Empirical formula ×n
n=molecular massEmperical frmular mass=9494=1
∴ Molecular formula=C6H6O
Similar Questions
A compound contains 32% carbon, 4% hydrogen and rest oxygen. Its vapour density is 75. Calculate the empirical and molecular formula.
View SolutionA compound contains 32% carbon, 4% hydrogen and rest oxygen. Its vapour density is 75. Calculate the empirical and molecular formula.
View SolutionA compound contains 32% carbon, 4% hydrogen and rest oxygen. Its vapour density is 75. Calculate the empirical and molecular formula.
View SolutionA compound contains 32% carbon, 4% hydrogen and rest oxygen. Its vapour density is 75. Calculate the empirical and molecular formula.
View SolutionAn organic compound containing carbon hydrogen and oxygen contains 52 .2 % carbon and 13.04 % hydrogen .Vapour density of the compound is 23 .Its molecular formula will be
View SolutionA compound has the following percentage composition by mass : Carbon = 54.55%, Hydrogen = 9.09% and Oxygen = 36.26%.
Its vapour density is 44. Find the Empirical and molecular formula of the compound.
(H = 1, C = 12,0 = 16)
View SolutionA compound is composed of 2.2% hydrogen, 26.6% carbon and 71.2% oxygen. Calculate the empirical formula of the compound. If its molecular mass is 90, find its molecular formula.
View SolutionA compound contains 52.2% C and 13 % hydrogen and remaining percentage oxygen. If vapour density of the compound is 46, molecular formula is
View Solution
