A compound on analysis gave the following percentage composition: Na=14.31% S = 9.97%, H = 6.22%, O = 69.5%, calcualte the molecular formula of the compound on the assumption that all the hydrogen in the compound is present in combination with oxygen as water of crystallisation. Molecular mass of the compound is 322 [Na = 23, S = 32, H = 1, 0 = 16]. Solution in Tamil
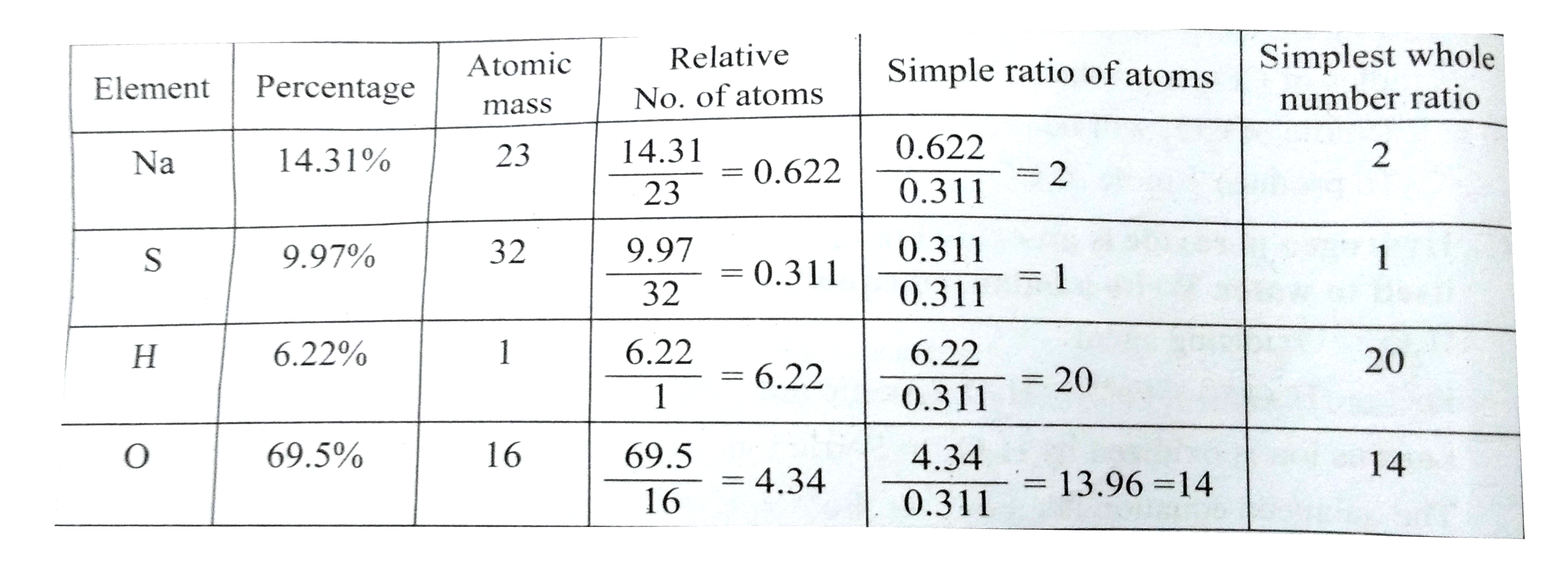
All H combines with 10 oxygen atoms to form as 10H2O
So the empirical formula is Na2SO4.10H2O
Empirical formula mass = (23×2)+(32×1)+(16×4)+(10×18)
= 46 + 32 + 64 + 180 = 322
n=Molecular massEmperical formula mass=322322=1
Molecular formula=Na2SO4.10H2O
Similar Questions
A compound on analysis gave the following percentage composition: Na=14.31% S = 9.97%, H = 6.22%, O = 69.5%, calcualte the molecular formula of the compound on the assumption that all the hydrogen in the compound is present in combination with oxygen as water of crystallisation. Molecular mass of the compound is 322 [Na = 23, S = 32, H = 1, 0 = 16].
View SolutionA compound on analysis gave the following percentage composition: Na=14.31% S = 9.97%, H = 6.22%, O = 69.5%, calcualte the molecular formula of the compound on the assumption that all the hydrogen in the compound is present in combination with oxygen as water of crystallisation. Molecular mass of the compound is 322 [Na = 23, S = 32, H = 1, 0 = 16].
View SolutionA compound on analysis gave the following percentage composition: Na=14.31% S = 9.97%, H = 6.22%, O = 69.5%, calcualte the molecular formula of the compound on the assumption that all the hydrogen in the compound is present in combination with oxygen as water of crystallisation. Molecular mass of the compound is 322 [Na = 23, S = 32, H = 1, 0 = 16].
View SolutionA compound on analysis gave the following percentage composition: Na=14.31% S = 9.97%, H = 6.22%, O = 69.5%, calcualte the molecular formula of the compound on the assumption that all the hydrogen in the compound is present in combination with oxygen as water of crystallisation. Molecular mass of the compound is 322 [Na = 23, S = 32, H = 1, 0 = 16].
View SolutionA compound on analysis was found to contain the following composition : Na=14.31%,S=9.97%,O=69.50 %and H=6.22 % Calculate the molecular formula of the compound assuming that the whole of hydrogen in the compound is present as water of crystallisation. Molecular mass of the compound is 322.
View SolutionA compound on analysis gave the following percentage composition. Na =14.31% , S= 9.97% , H= 6.22% , O=69.5% . The molecular mass of the substance is given as 322 Determine the molecular formula on the assumption that all the hydrogen atoms present in combination with oxygen as water of crystallisation : (AT. mass of Na =23 , H=1 , S=32, O=16)
View SolutionA compound on analysis gave the following percentage composition :
Na= 14.31%, S = 9.97%, H = 6.22%, O = 69.50%.
Calculate the molecular formula of the compound on the assumption that all the hydrogen in the compound is present in combination with oxygen as water of crystallisation. Molecular mass of the compound is 322.
(At. wt. of Na = 23, S = 32, H = 1, O = 16)
View SolutionA compound on analysis gave the following percentage composition by mass: H = 9.09, O = 36.36, C = 54.55. Molecular mass of compound is 88. Find its molecular formula.
View Solution

