Balance the following equations by oxidation number method
(i) Kr2Cr2O7+KI+H2SO4→K2SO4+Cr2(SO4)3+I2+H2O
(ii)KMnO4+Na2SO3→MnO2+Na2SO4+KOH
(iii)Cu+HNO3→Cu(NO3)2+H2O
(iv)KMnO4+H2C2O4+H2SO4→K2SO4+MnSO4+CO2+H2O
(i)Kr2Cr2O7+KI+H2SO4→K2SO4+Cr2(SO4)3+I2+H2O
Step 1
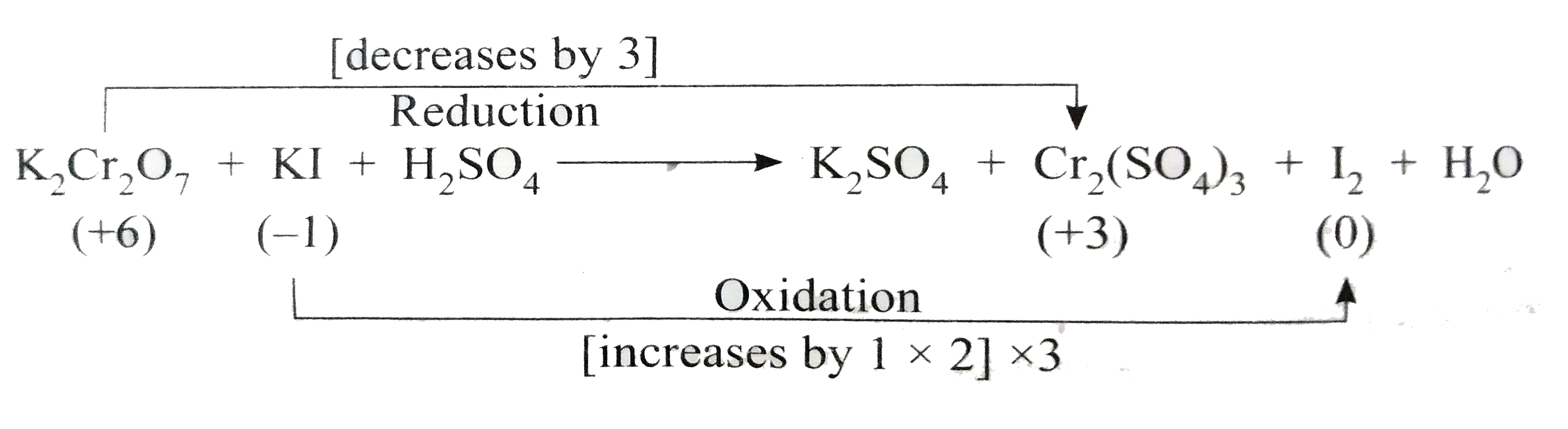
Step 2. K2Cr2O7+6KI+H2SO4→K2SO4+Cr2(SO4)3+3I2+H2O
Step 3. To balance other atoms
K2Cr2O7+6KI+H2SO4→4K2SO4+Cr2(SO4)3+3I2+H2O
Step 4. To balance oxygen and hydrogen, H2O and H2SO4 are multiplid by 7
K2Cr2O7+6KI+7H2SO4→4K2SO4+Cr2(SO4)3+3I2+7H2O
(ii) KMnO4+Na2SO3→MnO2+Na2SO4+KOH
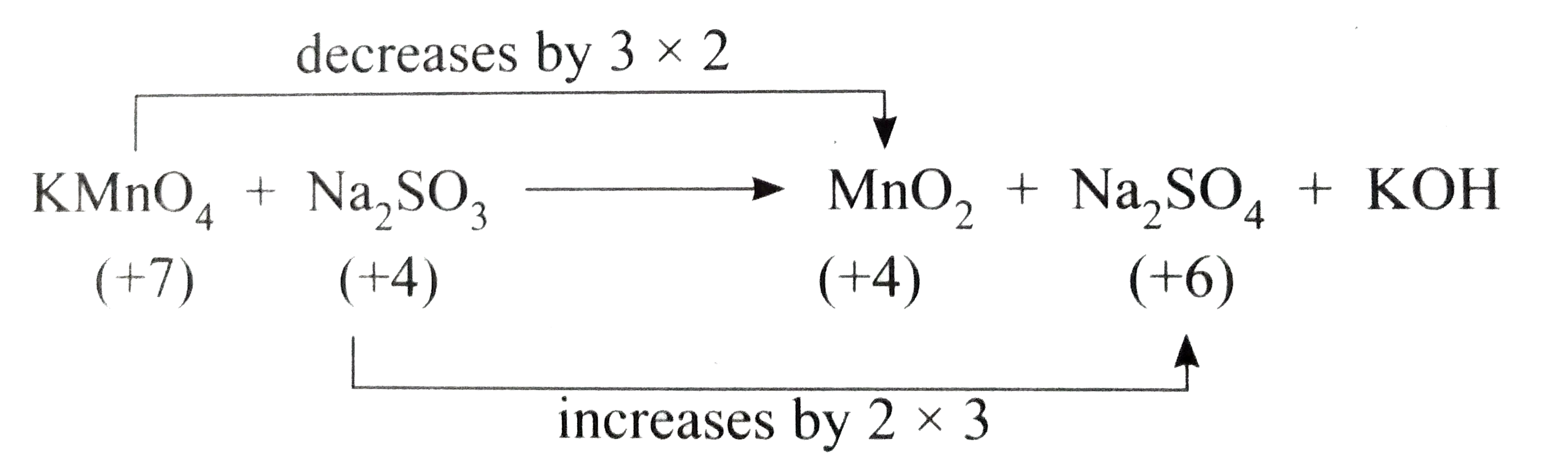
Stpe 2:2KMnO4+3Na2SO3→2MnO2+3Na2SO4+KOH
Step 3. balancing potassium KOH is multiplies by 2
2KMnO4+3Na2SO3→2MnO2+3Na2SO4+2KOH
Step 4. To balance H atom, H2O is added on reactant side.
2KMnO4+3Na2SO3+H2O→MnO2+3Na2SO4+2KOH
(iii) Cu+HNO3→Cu(NO3)2+NO2+H2O
Step 1.
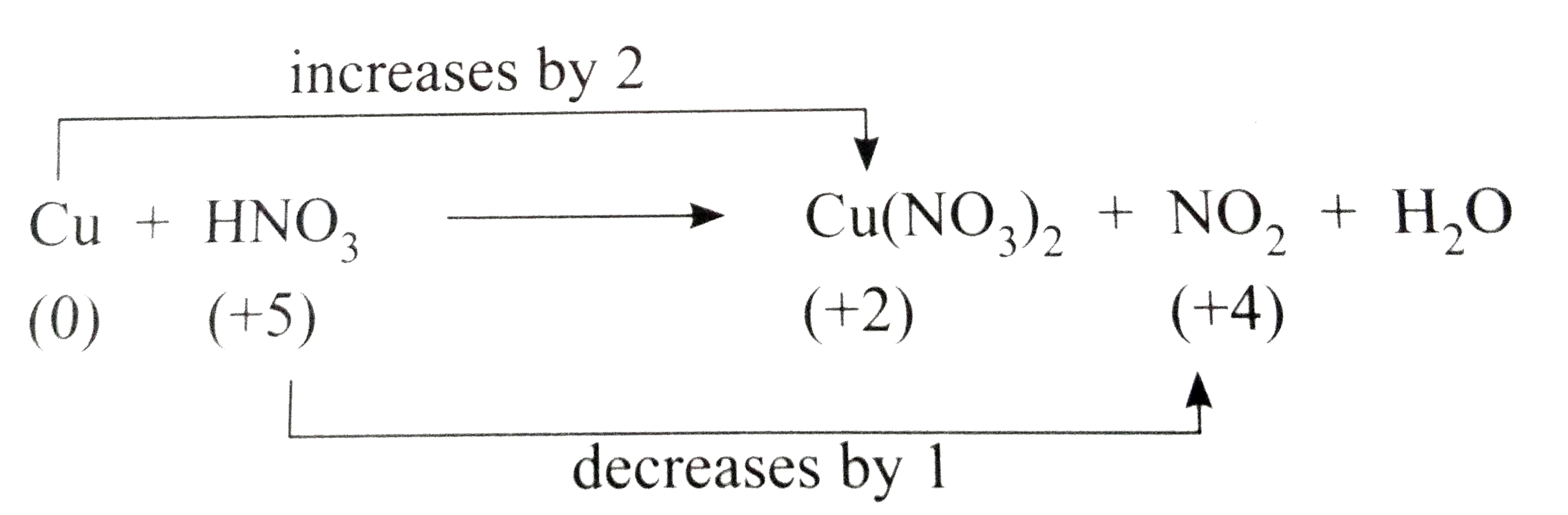
Stpe 2. Cu+2HNO3→Cu(NO3)2+NO2+H2O
Step 3. To balance Nitrogen 2HNO3 is multiplies by 2 and NO2 is multiplies by 2
Cu+4HNO3→Cu(NO)2+2NO2+H2O
Step 4. To balance oxygen, H2O is multiplied by 2
Cu+4HNO3→Cu(NO3)2+2NO2+2H2O
(iv) H2C2O4+KMnO4+H2SO4→K2SO4+MnSO4+CO2+H2O
Step 1
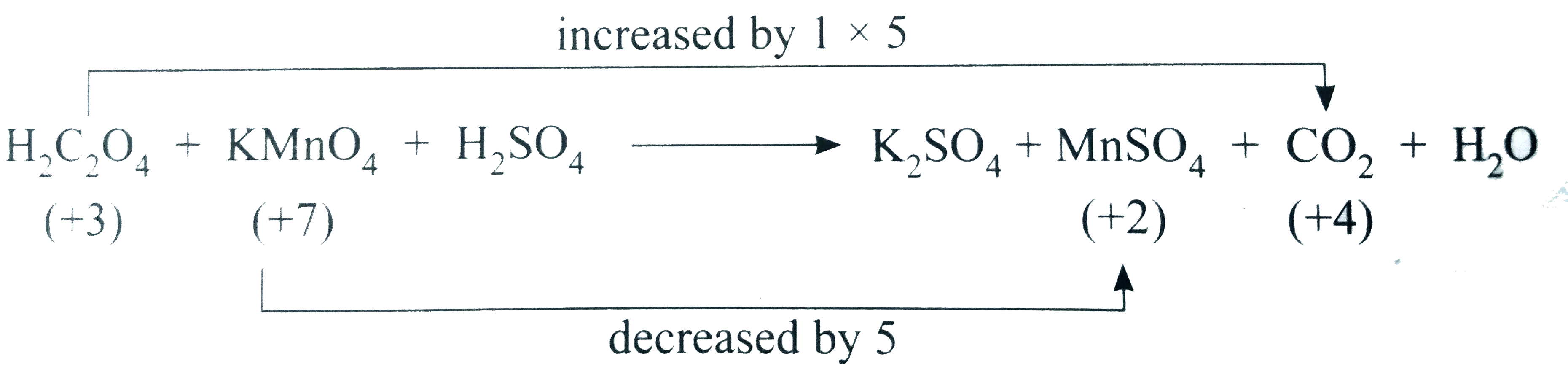
Step 2. 5H2C2O4+KMnO4+H2SO4→K2SO4+MnSO4+10CO2+H2O
Step3. To balance K, KMnO4 and MnSO4 are multiplied by 2
5H2Cr2O4+2KMnO4+H2SO4→K2SO4+2MnSO4+10CO2+H2O
Step 4. To balance O and H, H2O and H2SO4 are multiplies by 3 and 6
5H2C2O4+2MnSO4+10CO2+8H2O
Similar Questions
Balance the following equation by oxidation number method. KMnO_(4)+FeSO_(4)+H_(2)SO_(4)rarrK_(2)SO_(4)+MnSO_(4)+Fe_2(SO_(4))_(3)+H_(2)O
View SolutionKMnO_(4) + H_(2)SO_(4) + H_(2)C_(2)O_(4) rarr K_(2)SO_(4)+MnSO_(4) + CO_(2) + H_(2)O . Balance this equation.
View SolutionBalance the equation : SO_(2) + Na_(2)CrO_(4) + H_(2)SO_(4) rarr Na_(2)SO_(4) + Cr_(2)(SO_(4))_(3) + H_(2)O
View SolutionWhat is the mole coefficient of H2SO4 in the balanced equation.
K2Cr2O7+FeSO4+H2SO4→K2SO4+Fe2(SO4)3+Cr2(SO4)3+H2O
View SolutionBalance the following equation by oxidation number method:
K2Cr2O7+FeSO4+H2SO4→Cr2(SO4)3+Fe2(SO4)+K2SO4+H2O
View SolutionBalance the following chemical activity by ion-electron method- K_(2)Cr_(2)O_(7)+H_(2)SO_(4)+FeSO_(4)toFe_(2)(SO_(4))_(3)+Cr_(2)(SO_(4))_(3)+K_(2)SO_(4)+H_(2)O
View Solutionनिम्नलिखित अभिक्रिया में H2SO4 का तुल्यांकी भार है
Na2Cr2O7+3SO2+H2SO4→3Na2SO4+Cr2(SO4)3+H2O
View SolutionTaking the example of the reaction
K2Cr2O7+H2SO4+FeSO4→K2SO4+Cr2(SO4)3+Fe2(SO4)3+H2O,
explain how a redox reaction is balanced by oxidation number method.
View Solution

