Balance the following equation using oxidation number method
As2S3+HNO3+H2O→H3AsO4+H2SO4+NOSolution in Tamil
As2S3+HNO3+H2O→H3AsO4+H2SO4+NO
+3As2S3++5HNO3+H2O→+5H3AsO4+H2SO4++2NO
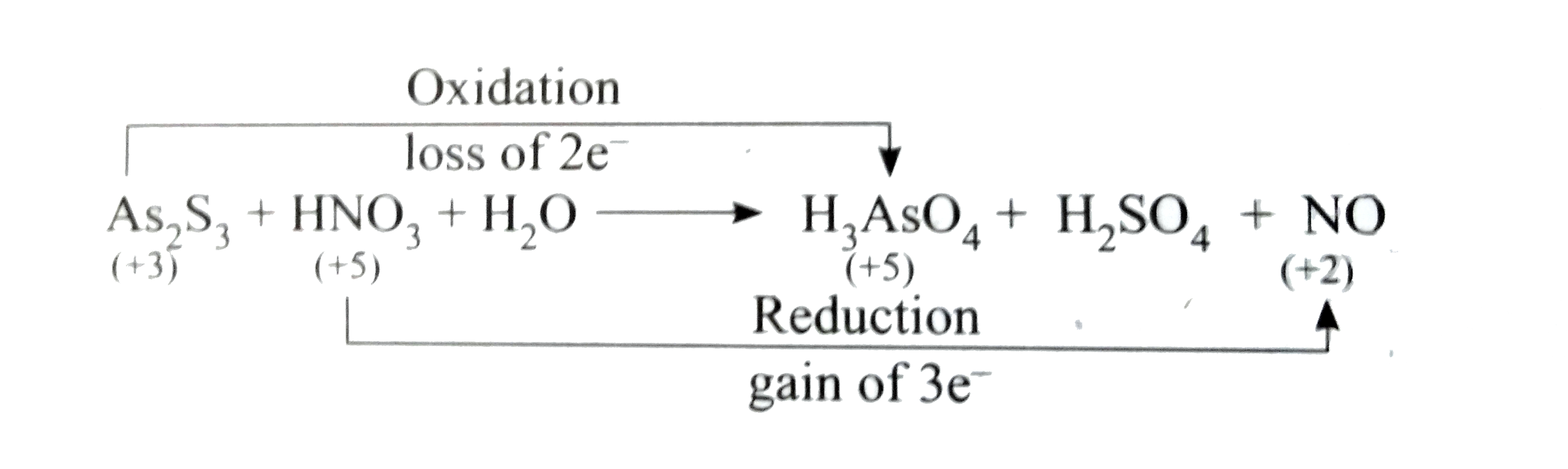
As2S3→H3AsO4+2e−×3
HNO3+3e−→NO×2
3As_(2)S_(3)rarr 3H_(3)AsO_(4)+cancel(6e^(-))
3As2S3+2HNO3→6H3AsO4+2NO
To balance oxygen and sulphur, H2O and H2SO4 are added.
3As2S3+2HNO3+H2O→6H3AsO4+2NO+H2SO4
3As2S3+28HNO3+4H2O→6H3AsO4+28NO+9H2SO4
Similar Questions
Balance the following equation using oxidation number method. S + HNO_(3) rarr H_(2)SO_(4) + NO_(2) + H_(2)O
View SolutionBalance the equation : As2S3+HNO3→H2AsO3+H2SO4+NO
View SolutionArsenic sulphide (As_(2)S_(3)) reacts with sulphuric acid (H_(2)SO_(4)) to form H_(3)AsO_(4) (Arsenic acid) and sulphur - dioxide (SO_(2)) . What will be the coefficient of H_(2)SO_(4),H_(3)AsO_(4)andSO_(2) respectively in the balanced reaction ?
View SolutionBalance the following equations by oxidation number method. KMnO_(4)+H_(3)AsO_(3)+HCl toKCl+MnCl_(2)+H_(3)AsO_(4)+H_(2)O
View SolutionAs_(2) S_(5) + HNO_(3) = H_(2) SO_(4) + NO_(2)+ H_(3) AsO_(4) + H_(2) O
View SolutionAs2S3+HNO3→H3AsO4+H2SO4+NO the element
which is oxidised in the reaction is / are
View SolutionThe number of modes of As_(2)S_(3) oxidised to H_(3)AsO_(4) " and " H_(2)SO_(4) by one mode of HNO_(3) (reduced to NO) is .
View SolutionBalance the following equations by oxidation number method.
P+HNO3→HPO3+NO+H2O
View Solution

