20.0 g of a magnesium carbonate sample decomposes on heating to give carbon dioxide and 8.0 g magnesium oxide. What will be the percentage of purity of magnesium carbonate in the sample?Solution in Tamil
60
84
75
96
The correct Answer is:B
MgCO3Δ−−→MgO+CO2↑⏐⏐
84 g of MgCO3 gives 40 g of MgO. (100% purity)
20g of MgCO3 will give =40×2084=9.52g
9.52g of MgO is given by 100% pure MgCO3.
8.0 g of MgO will be given by=100×89.52=84.03%
Topper's Solved these Questions
- BASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS Book:FULL MARKSChapter:BASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS Exercise:Additional Questions Solved(Match the following)
Explore 3 Videos
- BASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS Book:FULL MARKSChapter:BASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS Exercise:Additional Questions Solved(Fill in the blanks)
Explore 47 Videos
- BASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS Book:FULL MARKSChapter:BASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS Exercise:Textual Calculation based on Stoichiometry solved
Explore 6 Videos
- ALKALI AND ALKALINE EARTH METALSBook:FULL MARKSChapter:ALKALI AND ALKALINE EARTH METALSExercise:Additional Questions Solved
Explore 224 Videos
- BASIC CONCEPTS OF ORGANIC REACTIONS Book:FULL MARKSChapter:BASIC CONCEPTS OF ORGANIC REACTIONS Exercise:Additional Questions Solved (5-Marks Question)
Explore 10 Videos
Similar Questions
Match the List-I and List-II using the correct code given below the list.
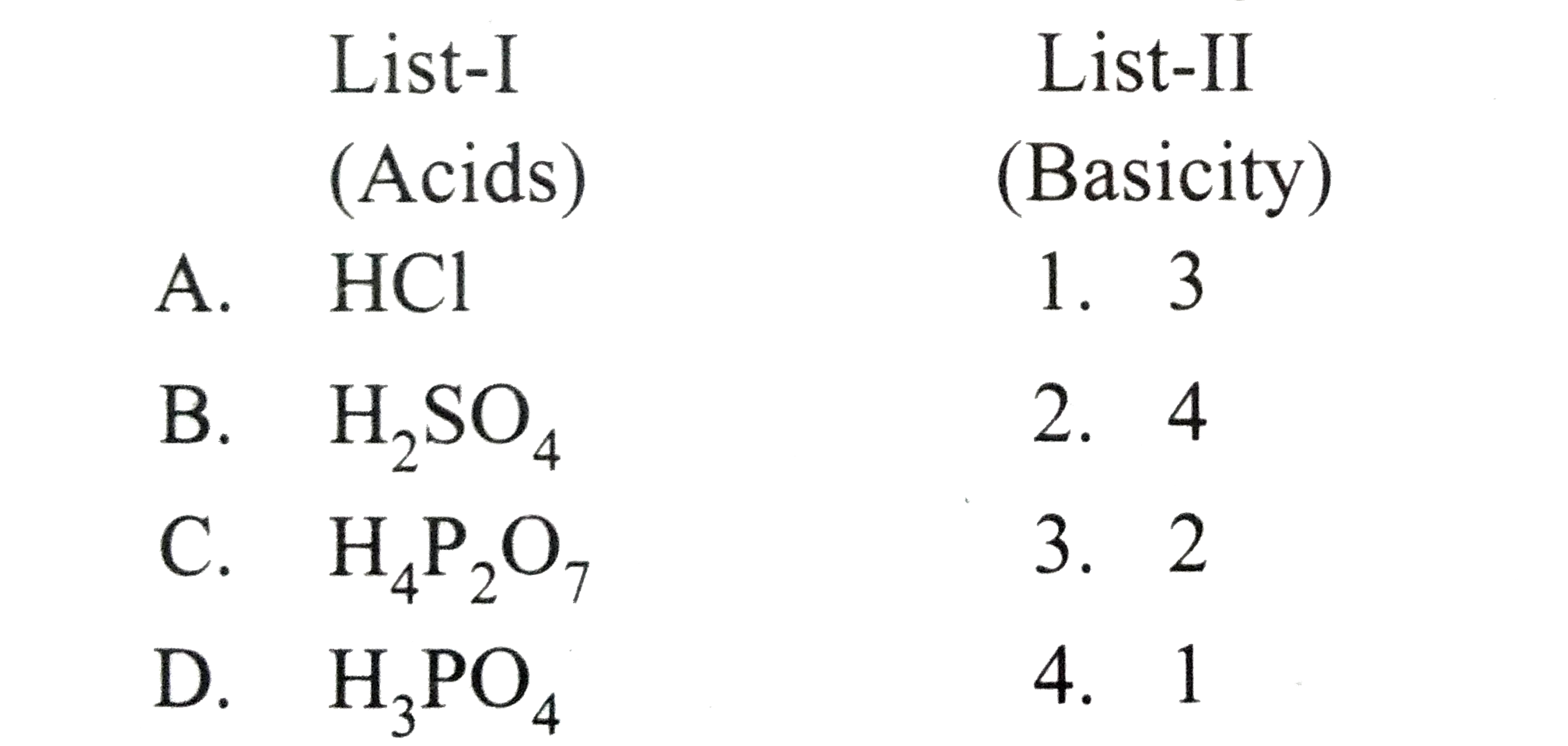
View SolutionOne mole of CO2 contains
View SolutionHow many moles of hydrogen is required to produce 10 moles of ammonia ?
View SolutionWhy alkali metals and alkaline earth metals are called s-block elements?
View SolutionExplain electron movement in organic reactions.
View Solution20.0 g of a magnesium carbonate sample decomposes on heating to give carbon dioxide and 8.0 g magnesium oxide. What be the percentage purity of magnsesium carbonate in the sample?
View Solution20.0 g of a magnesium carbonate sample decomposes on heating to give carbon dioxide and 8.0 g magnesium oxide. What will be the percentage purity of magnesium carbonate in the sample ? (At. Wt of Mg = 24)
View Solution20.0 g of magnisium carbonate sample decoposes on heating to give carbon dioxide and 8.0 g magnesium oxide. What will be the prcentage purity of magnesium carbonate in the sample?
View Solution20.0 g of magnesium carbonate sample decomposes on heating to give carbon dioxide and 8.0 g of magnesium oxide. What will be the percentage purity of magnesium carbonate in the sample?
View Solution20.0g of a magnesium carbonate sample decomposes on heating to give carbon dioxide and 8.0g magnesium oxide. What will be the percentage purity of magnesium carbonate in the sample (Mg=24)-
View Solution20.0 g of magnesium carbonate sample decomposes on heating to give carbon dioxide and 8.0 g of magnesium oxide. What will be the percentage purity of magnesium carbonate in the sample?
View Solution20.0 g मैग्नीशियम कार्बोनेट गर्म करने पर 8.0 g मैग्नीशियम ऑक्साइड देता है। मैग्नीशियम कार्बोनेट की शुद्धता का प्रतिशत है -
View Solution20 ग्राम मैग्नीशियम कार्बोनिट के नमूने को गर्म करने पर अपघटित होकर कार्बन डाइऑक्साइड एवं 8,0 ग्राम मैग्नीशियम ऑक्साइड देता हैं नमूने में मैग्नीशियम कार्बोनिट की शुध्दता का प्रतिशत क्या होगा ।
View Solution1g of an impure sample of magnesium carbonate (containing no thermally decomposable impurities ) on complete thermal decomposition gave 0.44g of carbon dioxide gas . The percentage of impurity in the sample is
View Solution1 g of an impure sample of magnesium carbonate (containing no thermally decomposable impurities) on complete thermal decomposition gave 0.44 g of carbon dioxide gas. The percentage of impurity in the sample is
View Solution
