An insecticide has the following percentage composition by mass. 47.5 % C, 2.54 % H and 50.0% Cl. Determine its empirical formula and molecular formulae. Molar mass of the substance is 354.5 gmol−1Solution in Tamil
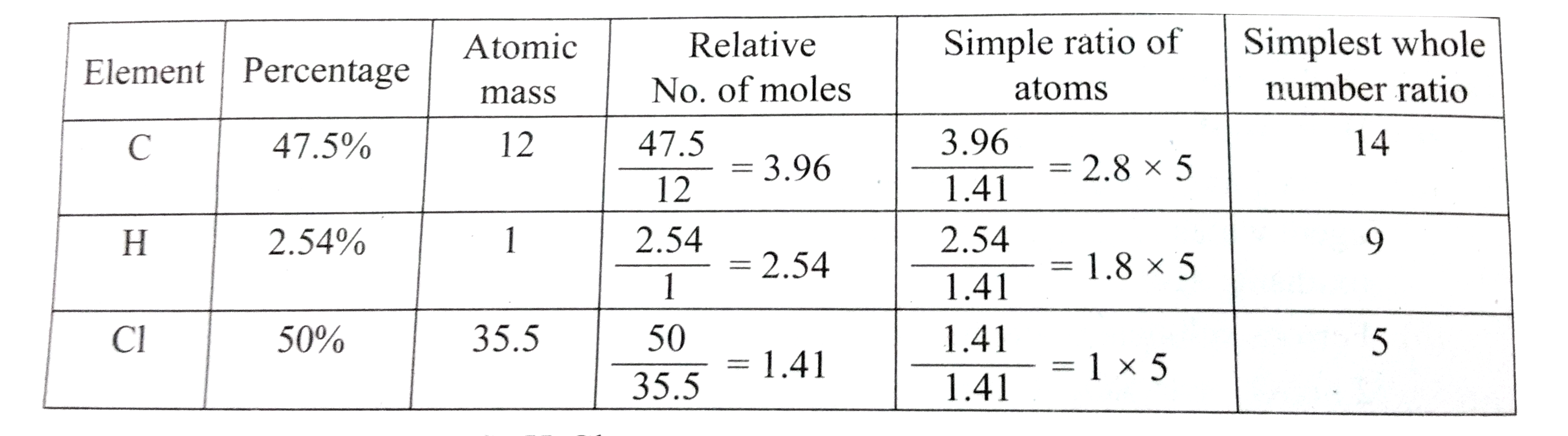
∴ The empirical formula is C14H9Cl5
Calculation of Molecular formula:
The empirical formula mass (C14H9Cl5)=(14×12)+(9×1)+(5×35.5)
168+9+177.5=354.5
n = Molecular massEmpirical formula mass=354.5354.5=1
Molecular formula=(Empirical formula)n
= (C14H9Cl5)
∴ Molecular formula = C14H9Cl5
Similar Questions
What is the simplest formula of the compound which has the following percentage composition: carbon 80%, Hydrogen 20%, If the molecular mass is 30, calcualte its molecular formula.
View SolutionWhat is the simplest formula of the compound which has the following percentage composition: carbon 80%, Hydrogen 20%, If the molecular mass is 30, calcualte its molecular formula.
View SolutionPercentage Composition|Empirical & Molecular Formula
View SolutionAn organic compound having C,H and O has 13.13 % H, 52.14% C. its molar mass is 46.068 gmol−1 . What are its empirical and molecular formulae ?
View SolutionThe compound A has the following percentage composition by mass : carbon 26. 7%, oxygen 71.1 %, hydrogen 2.2%. Determine the empirical formula of A. (Work to one decimal place) (H = 1, C = 12, O = 16). If the relative molecular mass of A is 90, what is the molecular formula of A ?
View SolutionThe molecular mass of benzene is 78 and its percentage composition is 92.3 C and 7.69 H. Determine the molecular formula of benzene?
View SolutionExplain the terms percentage composition, empirical formula and molecular formula.
View SolutionWhat is the simplest formula of the compound which has the following percentage composition carbon 80% , hydrogen 20% , if the molecular mass is 30. calculate its molecular formula ?
View Solution

