Which of the following property increases as we go down the group in the periodic property?Solution in Tamil
Ionization energy
Electronegativity
Atomic radius
Electron affinity
The correct Answer is:C
Step by step video, text & image solution for Which of the following property increases as we go down the group in the periodic property? by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- PERIODIC CLASSIFICATION OF ELEMENTS Book:FULL MARKSChapter:PERIODIC CLASSIFICATION OF ELEMENTS Exercise:Additional Questions(Match the follwing)
Explore 5 Videos
- PERIODIC CLASSIFICATION OF ELEMENTS Book:FULL MARKSChapter:PERIODIC CLASSIFICATION OF ELEMENTS Exercise:In-Text Question-Evaluate Yourself
Explore 7 Videos
- PHYSICAL AND CHEMICAL EQUILIBRIUMBook:FULL MARKSChapter:PHYSICAL AND CHEMICAL EQUILIBRIUMExercise:ADDITIONAL QUESTIONS SOLVED (NUMERICAL PROBLEMS)
Explore 3 Videos
Similar Questions
Match the following columns
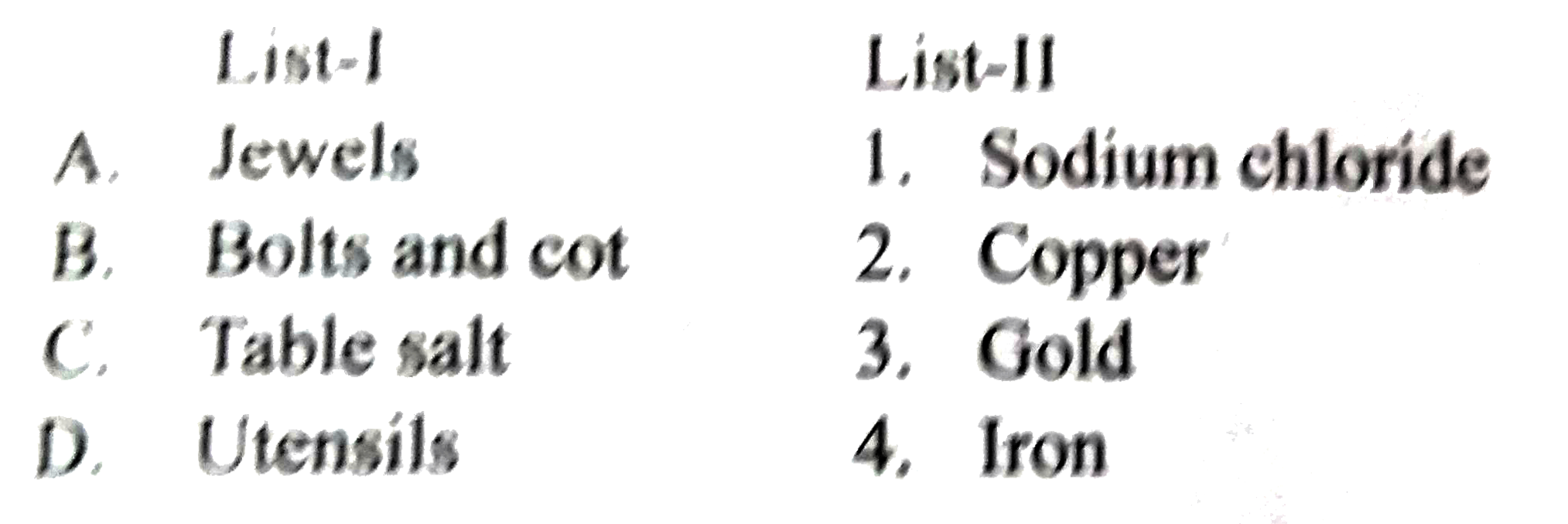
View SolutionWhat is the basic difference in approach between Mendeleev's periodic table and modern periodic table?
View SolutionFind the value of Kc for each of the following equilibria from the value of Kp
(a) 2NOCl(g)⇔2NO(g)+Cl2(g),KP=1.8×10−2 atm at 500 K
(b) CaCO3(s)⇔CaO(s)+CO2(g),KP=167 atm at 1073 K
View SolutionA property which progressively increases down a group in the periods table is :
View SolutionAll the elements in a group in the periodic table have the same
View SolutionA property which progressively increases down a group in the periodic table is :
View SolutionA property which progressively increases down a group in the periods table is :
View Solutiona) How does electropositivity of elements get affected as we move: (i) down a group (ii) across a period? b] Which atomic property increases as we move across a period and down a group?
View Solution
