Statement-I: Ionization enthalpy of nitrogen is greater than that of oxygen.
Statement-II: Nitrogen has exactly half filled electronic configuration which is more stable than electronic configuration of oxygen.Solution in Tamil
Statement-I is wrong but statement-Il is correct,
Statement-I is correct but statement-II is wrong.
Statement-I and I are correct and statement-Il is the correct explanation of statement-I.
Statement-I and II are correct but statement-II is not the correct explanation of statement I
The correct Answer is:C
Step by step video, text & image solution for Statement-I: Ionization enthalpy of nitrogen is greater than that of oxygen. Statement-II: Nitrogen has exactly half filled electronic configuration which is more stable than electronic configuration of oxygen. by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- PERIODIC CLASSIFICATION OF ELEMENTS BOOK - FULL MARKSCHAPTER - PERIODIC CLASSIFICATION OF ELEMENTS EXERCISE - Additional Questions(Match the follwing)
5 Videos
- PERIODIC CLASSIFICATION OF ELEMENTS BOOK - FULL MARKSCHAPTER - PERIODIC CLASSIFICATION OF ELEMENTS EXERCISE - In-Text Question-Evaluate Yourself
7 Videos
- PHYSICAL AND CHEMICAL EQUILIBRIUMBOOK - FULL MARKSCHAPTER - PHYSICAL AND CHEMICAL EQUILIBRIUMEXERCISE - ADDITIONAL QUESTIONS SOLVED (NUMERICAL PROBLEMS)
3 Videos
Similar Questions
Match the following columns
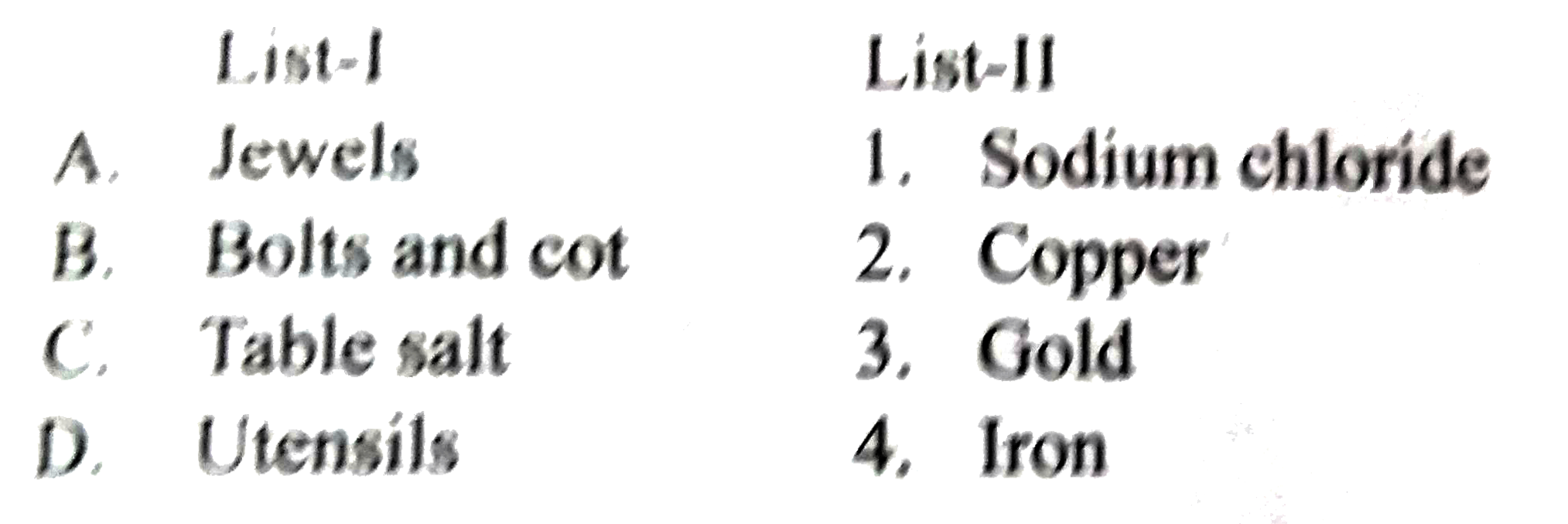
View SolutionWhat is the basic difference in approach between Mendeleev's periodic table and modern periodic table?
View SolutionFind the value of Kc for each of the following equilibria from the value of Kp
(a) 2NOCl(g)⇔2NO(g)+Cl2(g),KP=1.8×10−2 atm at 500 K
(b) CaCO3(s)⇔CaO(s)+CO2(g),KP=167 atm at 1073 K
View Solutionকেন নাইট্রোজেনের আয়নীভবন এনথ্যালপি অক্সিজেনের এনথ্যালপির থেকে বেশি?
View Solutionनाइट्रोजन की आयनन एन्थैल्पी ऑक्सीजन की तुलना में अधिक होती है, इस कारण -
View Solution(A) Electronegitivity of nitrogen is less than that of oxygen (R ) Nitrogen has stable P^(3) configuration where as oxygen doesnot have such a configuration
View SolutionNitrogen has positive electron gain enthalpy whereas oxygen has negative. However, oxygen has lower ionisation enthalpy than nitrogen. Explain.
View Solutionकथन : नाइट्रोजन की विद्युत ऋणता कार्बन से अधिक है।
कारण : नाइट्रोजन का स्थायी अर्धपूरित इलैक्ट्रॉनिक विन्यास होता है।
View Solution

