Suggest and explain an indirect method to calculate lattice enthalpy of sodium chloride crystal. Solution in Tamil
The Born-Haber cycle is used to determine the lattice enthalpy of NaCl as follows:
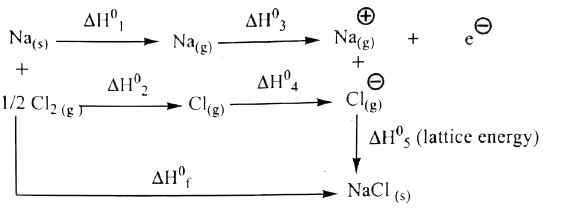
Formation of NaCl can be considered in 5 steps.
The sum of the enthalpy changes of these steps is equal to the enthalpy change for the overall reaction from which the lattice enthalpy of NaCl is calculated.
Na(s)+1/2Cl2(g)→NaCl(s) ΔH∘f=−411.3kJ mol−1
Sublimation : Na(s)→Na(g) ΔH01
Dissociation : 1/2Cl2(g)→Cl(g) ΔH02
Ionisation : Na(s)→Na+(g)+e− ΔH03
Electron affinity : Cl(g)+e−→Cl−(g) ΔH04
Lattice enthalpy : Na+(g)+Cl−(g)→NaCl(s) ΔH05 =?
ΔH0f=ΔH01+ΔH02+Δ03+Δ04+Δ05
ΔH05=ΔH0f−(ΔH01+ΔH02+ΔH03+ΔH04)
ΔH05 = Lattice enthalpy of NaCl .
By the above method , indirectly lattice enthalpy of NaCl is calculated , if the values of ΔH0f,ΔH01,ΔH02,ΔH03 and ΔH4%0 are given.
Topper's Solved these Questions
Similar Questions
In which of the following process, the process is always non-feasible?
View SolutionDefine "lattice energy" of crystals. Calculate the number of sodium ions and chloride ions per unit cell of NaCl.
View SolutionThe lattice enthalpy of an ionic compound is the enthalpy when one mole of an ionic compoud present in its gaseous state, dissociates into its ions. It is impossible to determine it directly by experiment. Suggest and explain in indirect method to measure lattice enthalpy of NaCl(s).
View SolutionThe lattice enthalpy of an ionic compound is the enthalpy when one mole of an ionic compound present in its gaseous state, dissociates into its ions. It is impossible to determine it directly by experiment. Suggest and explain an indirect method to measure lattice enthalpy of NaCI(s).
View SolutionExplain crystal structure of sodium chloride (NaCl).
View SolutionFor a crystal of sodium chloride, state : The structural arrangement of the sodium chloride crystal.
View SolutionFor a crystal of sodium chloride, state :
The structural arrangement of the sodium chloride crystal.
View SolutionFor a crystal of sodium chloride, state: (i) The type of lattice in which it crystallizes. (ii) The coordination number of each sodium ion and chloride ion in the crystal lattice. (iii) The number of sodium ions and chloride ions present in a unit cell of sodium chloride. (iv) The structural arrangement of the sodium chloride crystal.
View Solution
