Which of the following shows positive deviation from Raoult's law ? Solution in Tamil
Video Solution
Text Solution
(i) Let us consider the positive deviation shown by a solution of ethyl alcohl and water.
(ii) In this solution, the hydrogen bonding interaction between ethnol and water is weaker than those hydrogen bonding interactions amongst themselves (ethyl alcohol-ethyl alcohol and water-water interaction).
(iii) This results in the increased evaporation of both components from the aqueous solution of ethanol
(iv) Here, the mixing process is endothermic i.e., ΔHmixing>0 and there will bge a slight increase in volume (ΔVmixing>0)
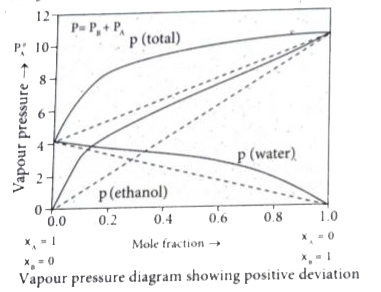
Vapout pressure diagram showing positive deviation
|
Topper's Solved these Questions
Similar Questions
(i) Define solution.
(ii) Explain the types of solutions with suitable example.
View SolutionCalculate the mole fraction of benzene is solution containing 30% by mass in carbon tetrachloride.
View SolutionWhich of the following liquid pairs shows a negative deviation from Raoult's law ?
View SolutionWhich of the following solutions shows positive deviation from Raoult's law ?
View SolutionPick out the combination which show positive deviations from Raoult.s law:
View SolutionWhich of the following liquid pairs shows a positive deviation from Raoult's law?
View SolutionWhich of the following does not show positive deviation from Raoult's law?
View SolutionThe mixture which shows positive deviation from Raoult's law is
View Solution
