Explain sp2 hybridisation in BF3.Solution in Tamil
sp2 hybridisation in boron trifluoride: Boron atom B:
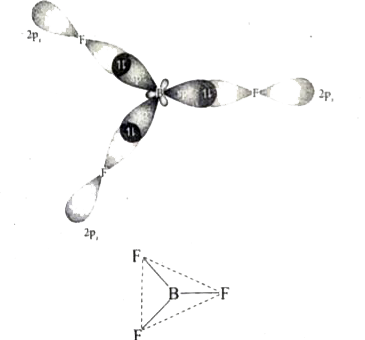
Electronic configuration [H2]2s2p1.

(ii) In boron, the s orbital and two p orbitals in the valence shell hybridises to generate three equivalent sp2 orbitals. These 3 orbitals lie in the same y plane and the angle between any two orbitals is equal to 120∘
(iii) The 3 sp2 hybridised orbitals of boron now overlap with the 2pz orbitals of fluorine (3 atoms)
This overlap takes place along the axis.
Topper's Solved these Questions
Similar Questions
Draw the lewis structures for
(i) Nitrous acid (HN02) (ii) Phosphoric acid (iii) Sulphur troxide (SO3)
View SolutionExplain about Kossel-Lewis approach to chemical bonding.
View SolutionThe hybridisation in BF_(3) is ________.
View SolutionIn which of the following molecules / ions BF_(3), NO_(2)^(-), H_(2) the central atom is sp^(2) hybridised ?
View SolutionExplain sp^(2) hybridisation in BF_(3) .
View SolutionCalculate the hybridisation of BF3
View SolutionExplain Sp2 hybridisation taking boron trichloride (BCl3) as an example.
View SolutionExplain sp3 hybridisation by taking methanae as example.
View SolutionExplain sp2 hybridization BF3 molecule.
View SolutionExplain sp hybridisation with an example.
View SolutionIn which of the following molecules / ions BF3,NO−2,H2 the central atom is sp2 hybridised ?
View SolutionCalculate the hybridisation of BF3
View SolutionExplain sp3 d hybridisation with a suitable example.
View SolutionExplain about sp^(3)d^(2) hybridisation with an example.
View SolutionThe hybridisation in BF3 is ________.
View Solution
