Draw and explain the M.O. diagram of lithium molecule.
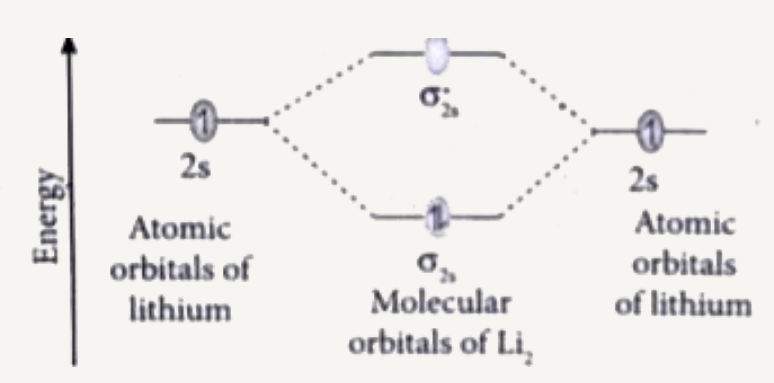 Solution in Tamil
Solution in Tamil
(i) Electronic configuration of Li atom: 1s2 2s1
(ii) Electronic configuration of Li2 molecule is σ1s2 1s2 σs2
(iii) Bond order =Nb−Na/2=4−2/2
(iv) Li2 molecule has no unpaired electrons, hence it is diamagnetic.
Topper's Solved these Questions
Similar Questions
Explain about Kossel-Lewis approach to chemical bonding.
View Solutionअमीटर की संरचना आरेख खींचकर समझाइये ।
View SolutionDraw the M.O diagram for oxygen molecule calculate its bond order and show that O2 is paramagnetic.
View Solutionविधुत परिपथ का व्यवस्था आरेख खींचिये और उसको समझाइए।
View SolutionWirte the energy level diagram of lithium molecule.
View SolutionDraw the M.O diagram for oxygen molecule calculate its bond order and show that O2 is paramagnetic.
View SolutionDraw the M.O diagram for oxygen molecule and calculate its bond order and show that O_(2) is paramagnetic.
View SolutionDrew the M.O diagram of oxygen molecule. Calculate its bond order and magnetic character.
(ii) Draw and explain the graph obtained by plotting solubility versus temperature for calcium chloride.
View SolutionDraw and explain electric circuit diagram.
View SolutionDraw. Lewis dot diagrams for the following
Lithium Fluoride (LiF)
View SolutionThe covalent bond can be explained by molecular orbital theory.Using M.O. diagram,Explain the paramagnetic nature of oxygen molecule.
View SolutionDraw the diagram of Electrolytic cell and explain.
View SolutionDraw the molecular orbital diagram of O_2 molecule. How would you explain the paramagnetic nature of the molecule on the basis of this diagram?
View SolutionDraw the diagram of monohybrid cross and explain it.
View SolutionDraw orbital diagrams of Fluorine molecule
View Solution

