Explain the mechanis of addtion of HBr to 3 methyl 1- butene
Text Solution
Consider the addition of HBr to 3- methyl 1- butene Here the expected product according to Markovanikoff.s rule is 2- bromo 3- methylbutane but the actual major product is 2- bromo 2- methylbutane . This is because the secondary teh satble tertiary carbocation attack of Br∘ on this teritiary carbocation gives the major product 2 - bromo 2- methylbutane

|
Similar Questions
Knowledge Check
Addition of HCl to 2-methyl-2-butene mainly gives
A1-chloro-2-methylbutaneB2-chloro-2-methylbutaneC2-chlorobutaneD1-chlorobutaneThe addtion of Br3 to trans-2-butene produces
A(+)2,3-dibromobutaneB(-)2,3=dibromobutaneCrac-2,3-dibromobutaneDmeso-2,3-dibromobutaneConsider the addition of HBr to 3,3-Dimethyl-1-butene shown below. What is the best mechanistic explanation for the formation of the observed product?
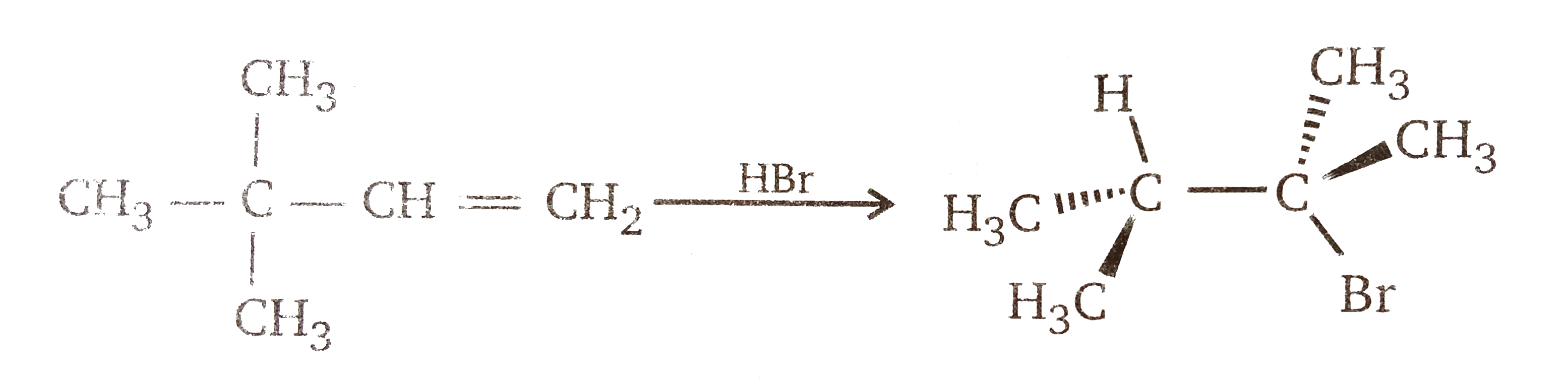 AProtonation of the alkene followed by a hydride shift and addition of bromide to the carbocationBDouble bond shift in the alkene following by the protonation and addition of bromide to the carbocation.CAddition of bromide to the alkene followed by a double bond shift and protonationDProtonation of the alkene followed by a methyl shift and addition of bromide to the carbocation
AProtonation of the alkene followed by a hydride shift and addition of bromide to the carbocationBDouble bond shift in the alkene following by the protonation and addition of bromide to the carbocation.CAddition of bromide to the alkene followed by a double bond shift and protonationDProtonation of the alkene followed by a methyl shift and addition of bromide to the carbocation
2-ethyl-3-methyl-1-butene
View SolutionExplain the ozonolysis of 2-butene.
View SolutionHow many of the following alkenes on addition of HBr would give the sae product in the presence or absence of peroxide propene, 1-butene, 2-butene, 2-methylpropene, 3-methyl-1-butene, 2,3-dimethyl-1-butene, 2-pentene, 1-pentene, 4-methyl-2-pentene
View SolutionHow many of the following alkenes on addition of HBr would give the sae product in the presence or absence of peroxide propene, 1-butene, 2-butene, 2-methylpropene, 3-methyl-1-butene, 2,3-dimethyl-1-butene, 2-pentene, 1-pentene, 4-methyl-2-pentene
View SolutionWhich of the following are formed on addition reaction of DCl with 3-methyl-1-butene
View Solution

