Which alkyl halide from the following pair is (i) chiral (ii) undergoes faster SN2 reaction?

Text Solution

CH3−H∣C∗∣Br−CH3−CH3. Here the C∗ is chiral carbon atom and it is surrounded by four different groups.
 i.e., I-chlorobutane undergoes faster SN2 reaction. CH3−CH2−CH2Cl is a primary alkyl halide and so it undergoes faster SN2 reaction.
i.e., I-chlorobutane undergoes faster SN2 reaction. CH3−CH2−CH2Cl is a primary alkyl halide and so it undergoes faster SN2 reaction.
|
Topper's Solved these Questions
Similar Questions
Which of the following is an example for polyhalo compounds?
View SolutionThe alkyl halide that undergoes S_(N)I reaction more readily is :
View SolutionOut of
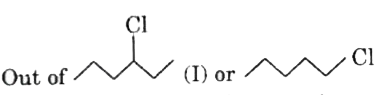 the compound I/II undergoes faster SN1 reaction.
the compound I/II undergoes faster SN1 reaction.
View SolutionWhich compound in the following pair undergoes faster SN1 reaction?
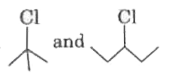
View SolutionOut of
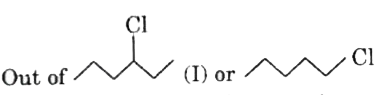 the compound I/II undergoes faster SN1 reaction.
the compound I/II undergoes faster SN1 reaction.
View SolutionWhich compound in the following pair undergoes faster S_(N)1 reaction?
View SolutionWhich alkyl halide from the following pair is (i) Chiral and (ii) undergoes S_(N)1 reaction faster ? (a) (CH_(3))_(3)CBr (b) CH_(3)CH_(2)CHBrCH_(3)
View Solution(i) Which alkyI hailde from the following pair is chiral and undergoes faster SN2 reaction ?

(ii) Out of SN1andSN2 which reaction occurs with
(a) Inversion of configuration
(b) Recemisation
View Solution

