What is Sabatier - Sendersens reaction?
The process of addition of H2 to unsaturated compounds ( alkenes or alkynes ) in known as hydrogenation . The above process can be cataysed by nikel at 298 K . This reaction is known as sabatier - sendersens reaction for example :
( Ethene)CH2=CH2+H2Ni−−→298KCH3−CH3(Ethane )
Topper's Solved these Questions
Similar Questions
Knowledge Check
Which of the following hydrocarbons cannot be obtained Sabatier and Senderens reaction
ACH4BC2H6CC3H8DAllA hydrocarbon containing 2 carbon atoms gives Sabatier and Senderens reaction but does not give precipitate with ammoniacal silver nitrate solution. The hydrocarbon in the question is
AEthaneBAcetyleneCEthyleneDNone of theseHydrogenation of alkenes and alkynes takes place in presence of certain catalysts. In sabatier-Senderen's reaction, the addition of hydrongen taken place in presence of nickel catalyst. Controlled hydrogenation of alkyne in presence of Lindlar's catalyst gives cis-alkene. Non terminal alkynes are reduced by Na or Li metal dissolved in liquid NH3. In this reaction trans alkenes are formed.
Q. In which of the following the reaction is most exothermicA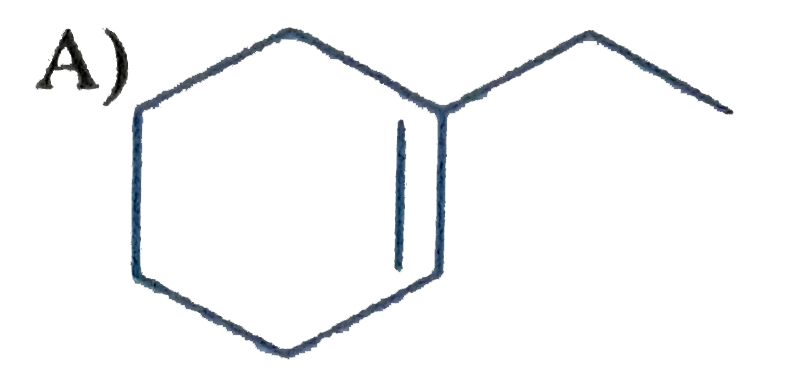 B
B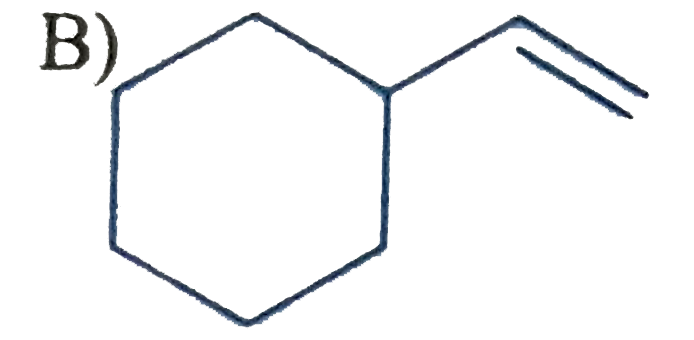 C
C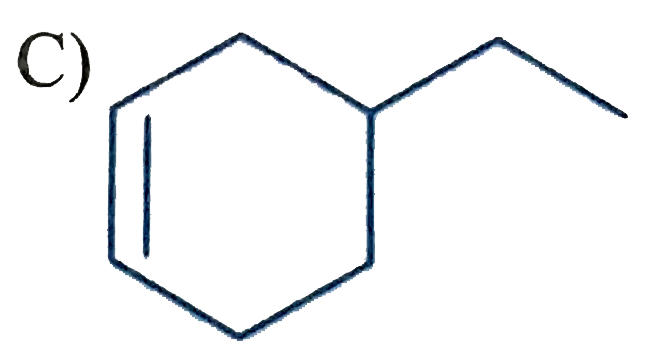 D
D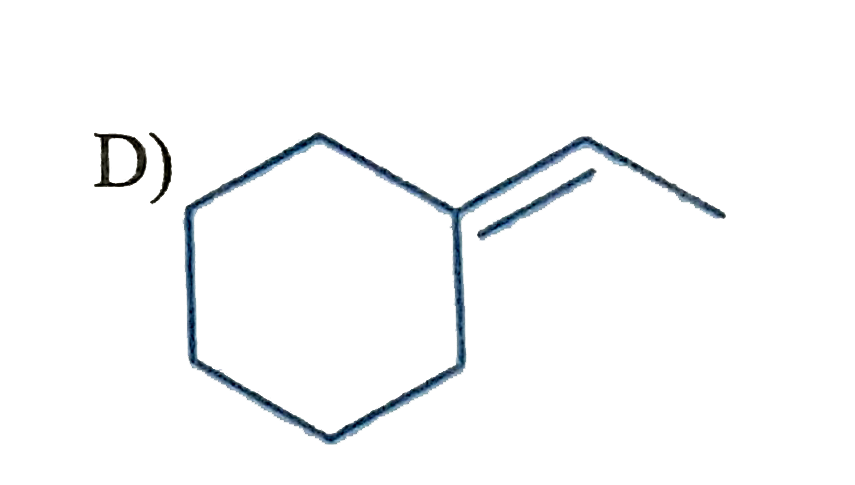
Sabatier-Senderen's reduction.
View SolutionHydrogenation of alkenes and alkynes takes place in presence of certain catalysts. In sabatier-Senderen's reaction, the addition of hydrongen taken place in presence of nickel catalyst. Controlled hydrogenation of alkyne in presence of Lindlar's catalyst gives cis-alkene. Non terminal alkynes are reduced by Na or Li metal dissolved in liquid NH3. In this reaction trans alkenes are formed.
Q. The product of the following reaction is
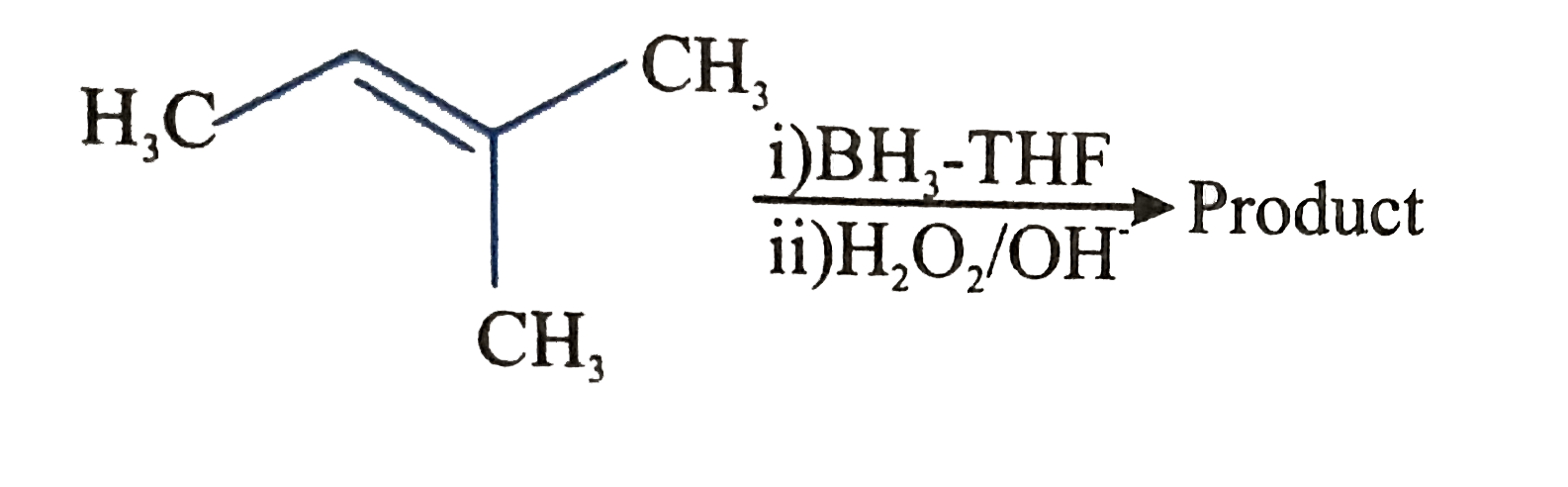
View SolutionHydrogenation of alkenes and alkynes takes place in presence of certain catalysts. In Sabatier Senderen's reaction, the addition of hydrogen takes place I presence of Raney nickel catalyst. Platininm and palladium can also be used as catalyst and used in finley divided state. Experimentally, it is observed that less crowded alkenes adsorb H2 with faster rate. Controlled hydrogenation of alkyne H2 with faster rate. Controlled hydrogenation of alkyne in presence of Lindlar's catalyst yields cis product i.e., 'cis' alkene. Thus, in presence of Lindlar's catalyst 'syn' addition takes place. The relative rate of hydrogenation follows the order:
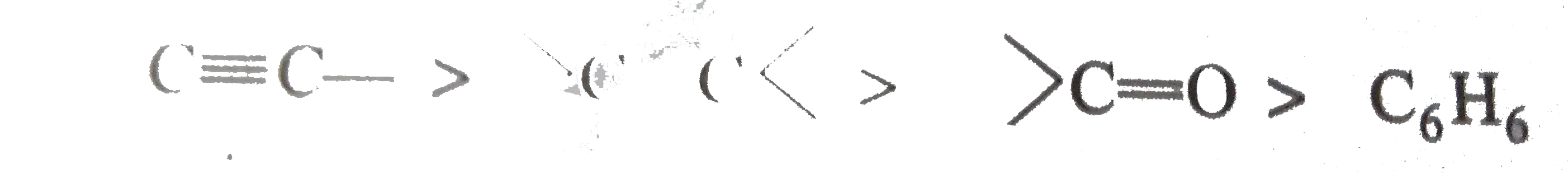
Non-terminal alkynes are reduced in presence of Na or Li metal dissolved in liquid ammonia. In this reaction, anti-addition of hydrogen results into the trans-product.
Answer the following questions:
The relative rate of catalytic hydrogenation of following alkenes is:
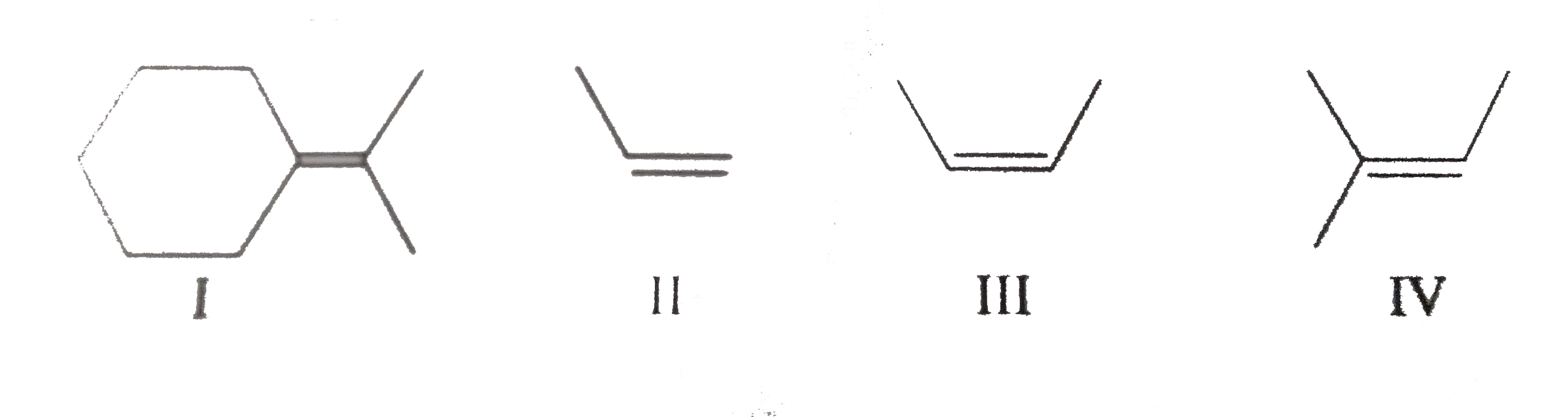
View SolutionThe Sabatier-Senderens reduction is carried out by passing the vapors of organic compound mixed with hydrogen over _______ catalyst at 200−300∘C.
View SolutionAmtch List I with List I and select the correct answer from the given codes
List IList IIReaction Metals used A. Wurtz reaction 1.NiB.Sabatier senderen's reaction 2. Znc. Frankland reaction e. LiD. Corey -House synthesis 4. Na
View Solution

