Name the products formed in the following reactions
( i) Reaction of sodium nitrate with ammonium sulphate
(ii) Reaction of potasium iodic with nitric acid
(iii) Addition of HNO3 to sulphur
NA
Topper's Solved these Questions
- SOME IMPORTANT ELEMENTS AND THEIR COMPOUNDSBook:PEARSON IIT JEE FOUNDATIONChapter:SOME IMPORTANT ELEMENTS AND THEIR COMPOUNDSExercise:Essay type question
Explore 5 Videos
- SOME IMPORTANT ELEMENTS AND THEIR COMPOUNDSBook:PEARSON IIT JEE FOUNDATIONChapter:SOME IMPORTANT ELEMENTS AND THEIR COMPOUNDSExercise:Level-1
Explore 44 Videos
- SOME IMPORTANT ELEMENTS AND THEIR COMPOUNDSBook:PEARSON IIT JEE FOUNDATIONChapter:SOME IMPORTANT ELEMENTS AND THEIR COMPOUNDSExercise:Level-3
Explore 10 Videos
- MOCK TEST Book:PEARSON IIT JEE FOUNDATIONChapter:MOCK TEST Exercise:Question
Explore 5 Videos
- SOME NATURAL PHENOMENABook:PEARSON IIT JEE FOUNDATIONChapter:SOME NATURAL PHENOMENAExercise:COMPETITION CORNER
Explore 37 Videos
Similar Questions
Write the names and structures of the products formed by the following reactions :
( i) addition of HCl to acetone .
(ii ) Reaction of semicarbazide with formaldehyde .
(iii ) Addition of Grignard reagent to butanone
(iv ) Reaction of acetophenone with hydrazine in strong base .
(v ) Reaction of PCl5 and propanone
View SolutionWrite the balanced ionic equation for the reaction of sodium bicarbonate with sulphuric acid
View Solution
Knowledge Check
The metal which does not form ammonium nitrate by reaction with dilute nitric acid is
AAlBFeCPbDMgThe metal which does not form ammonium nitrate by reaction with dilute nitric acid is
AAlBFeCPbDMgThe following data are for the decomposition of ammonium nitrate in aqueous solution :
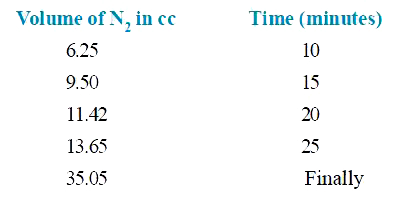
The order of the reaction is:AZeroBOneCTwoDThree
Write the following reactions:
(i) Swarts reaction
(ii) Sandmeyer reaction
(iii) Wurtz-Fitting reaction
View SolutionGive the product of the following reactions:
a.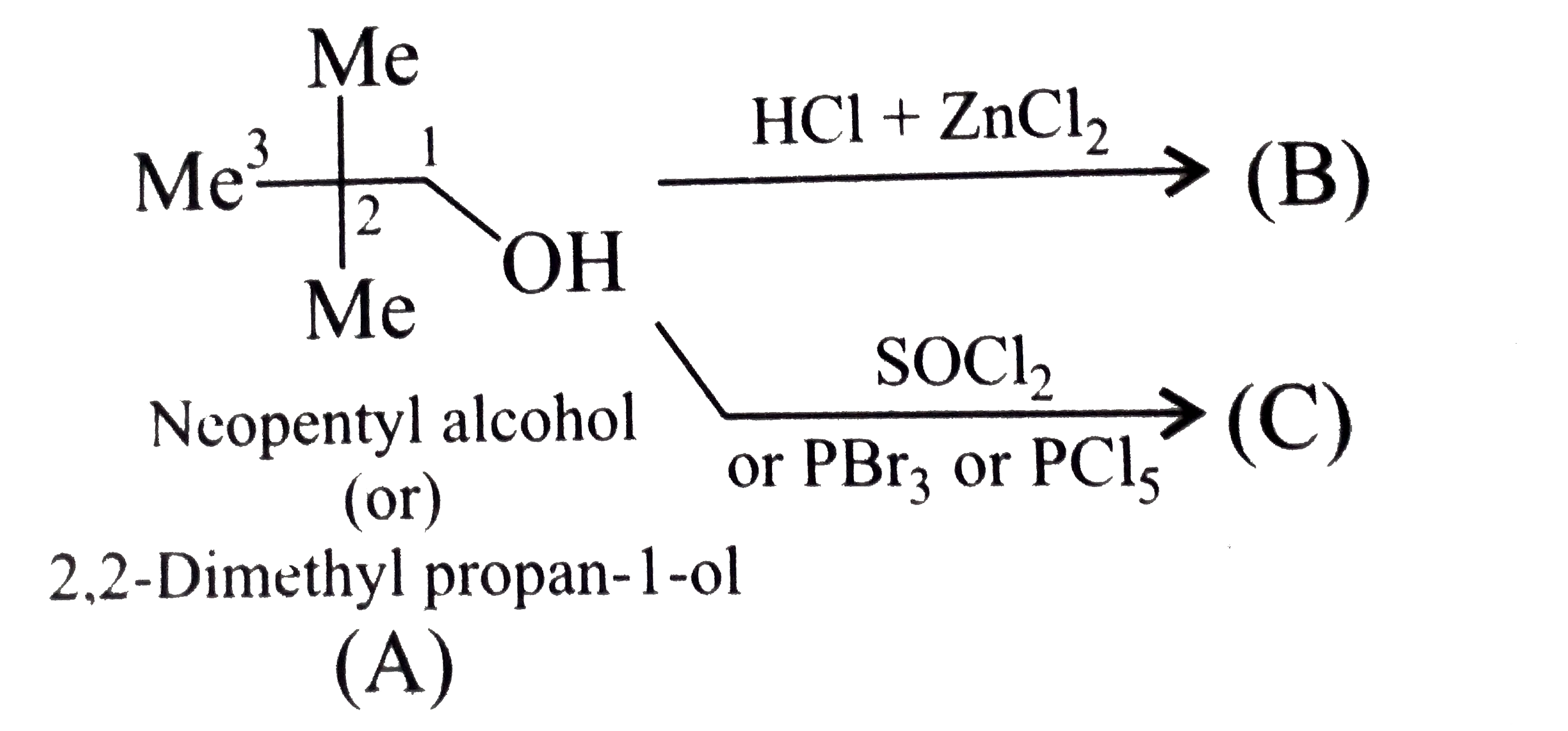
b. Given the stereochemical or rerranfged product of the following reaction.
i.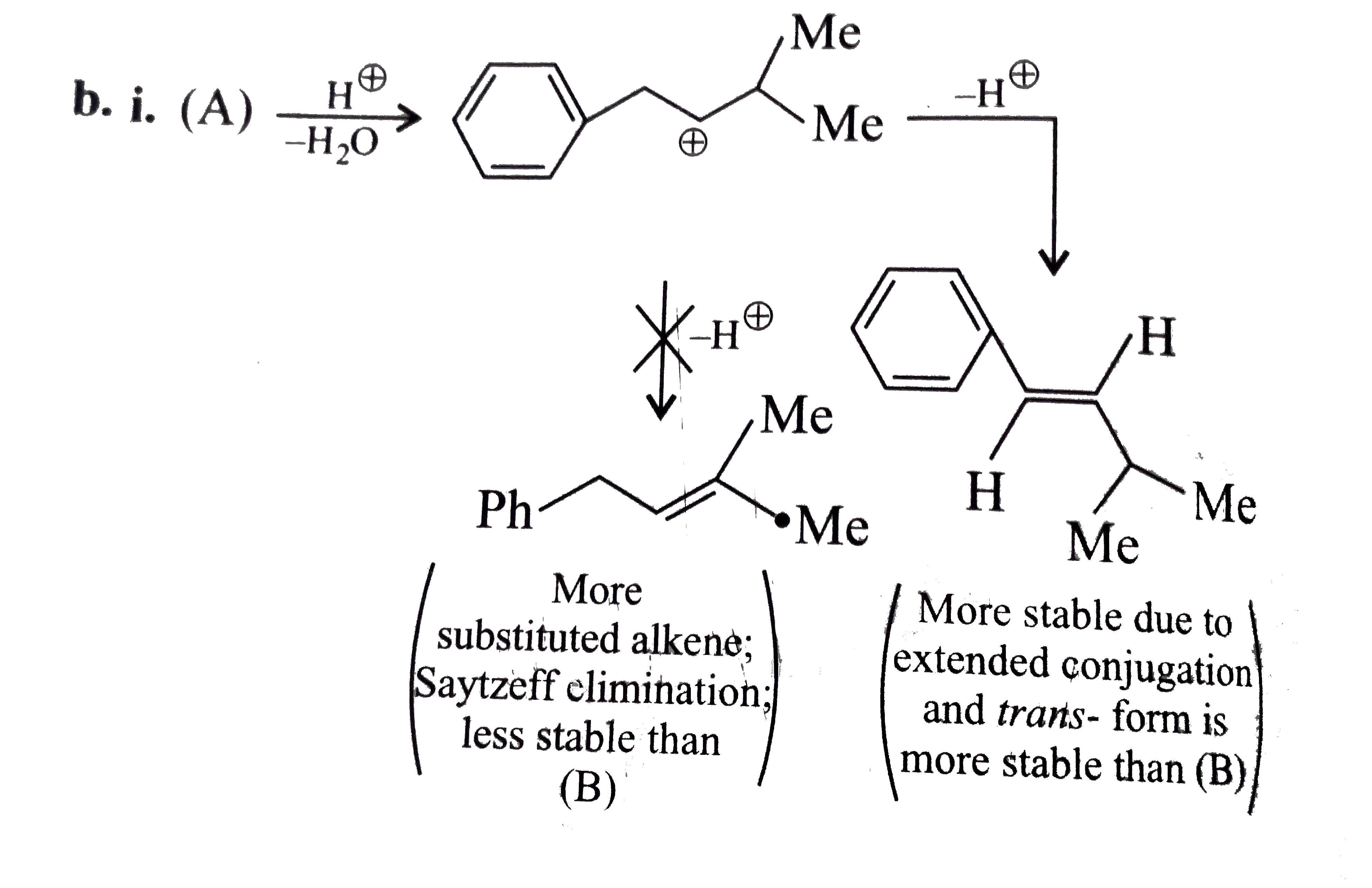
ii.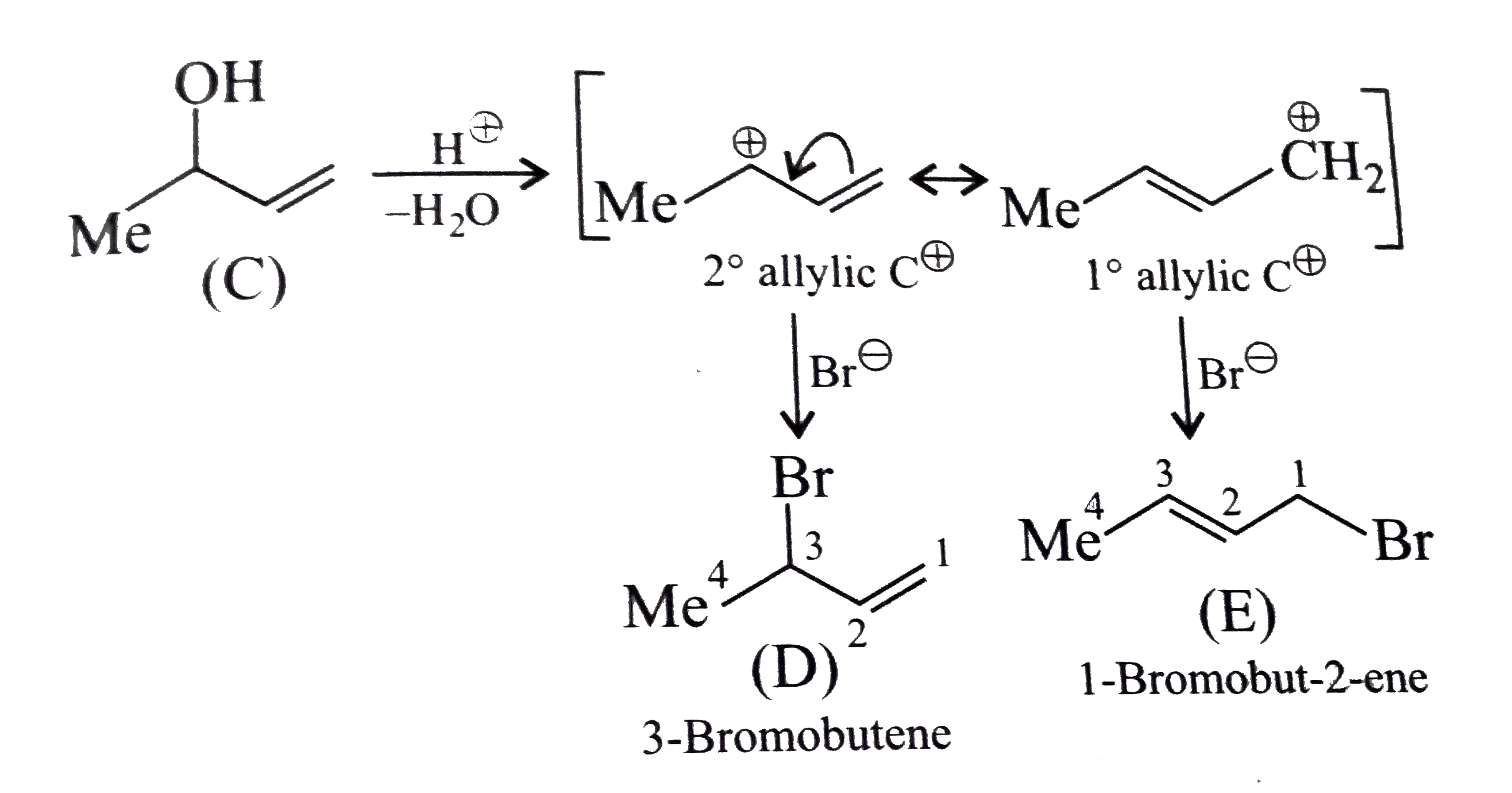
iii.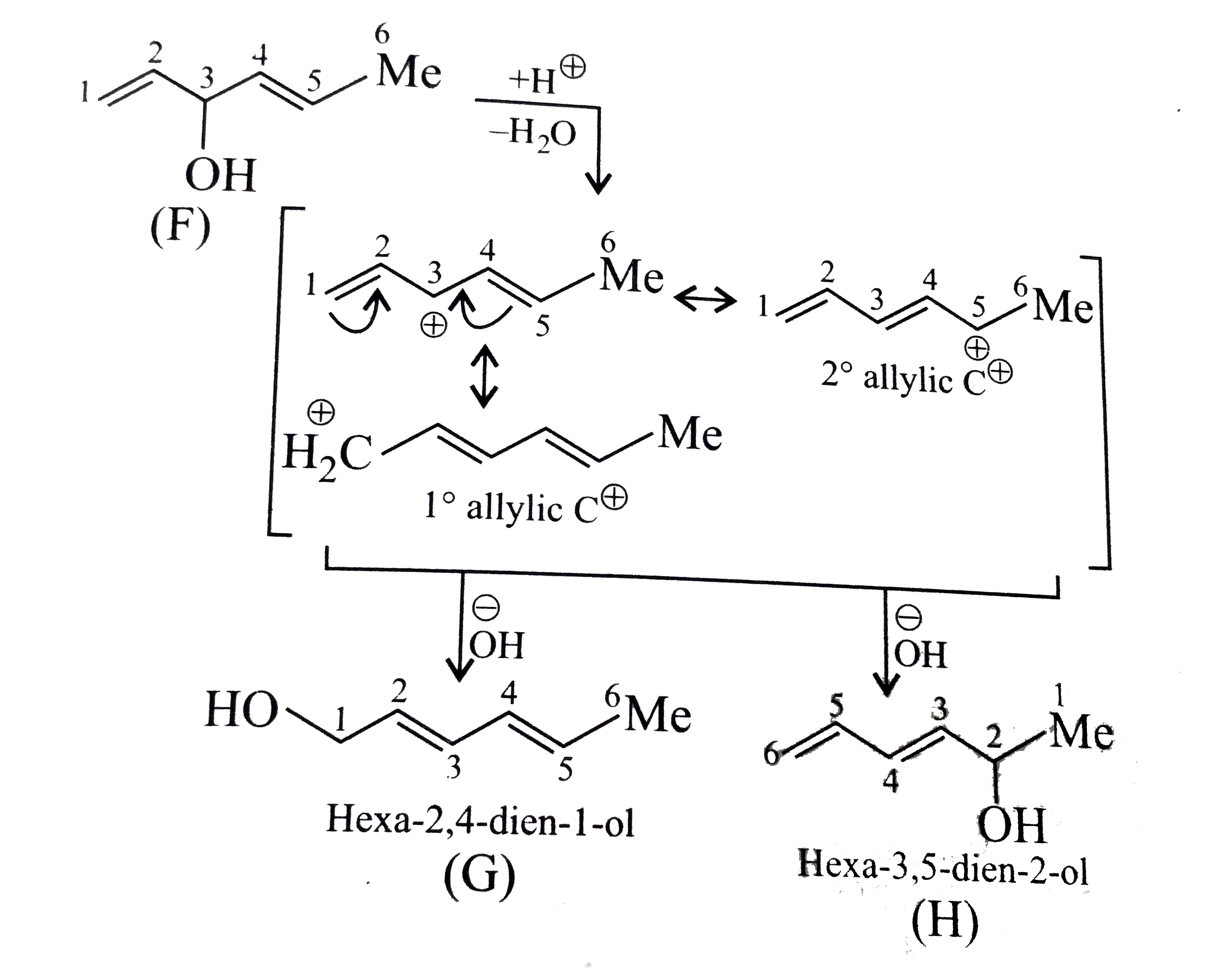
iv.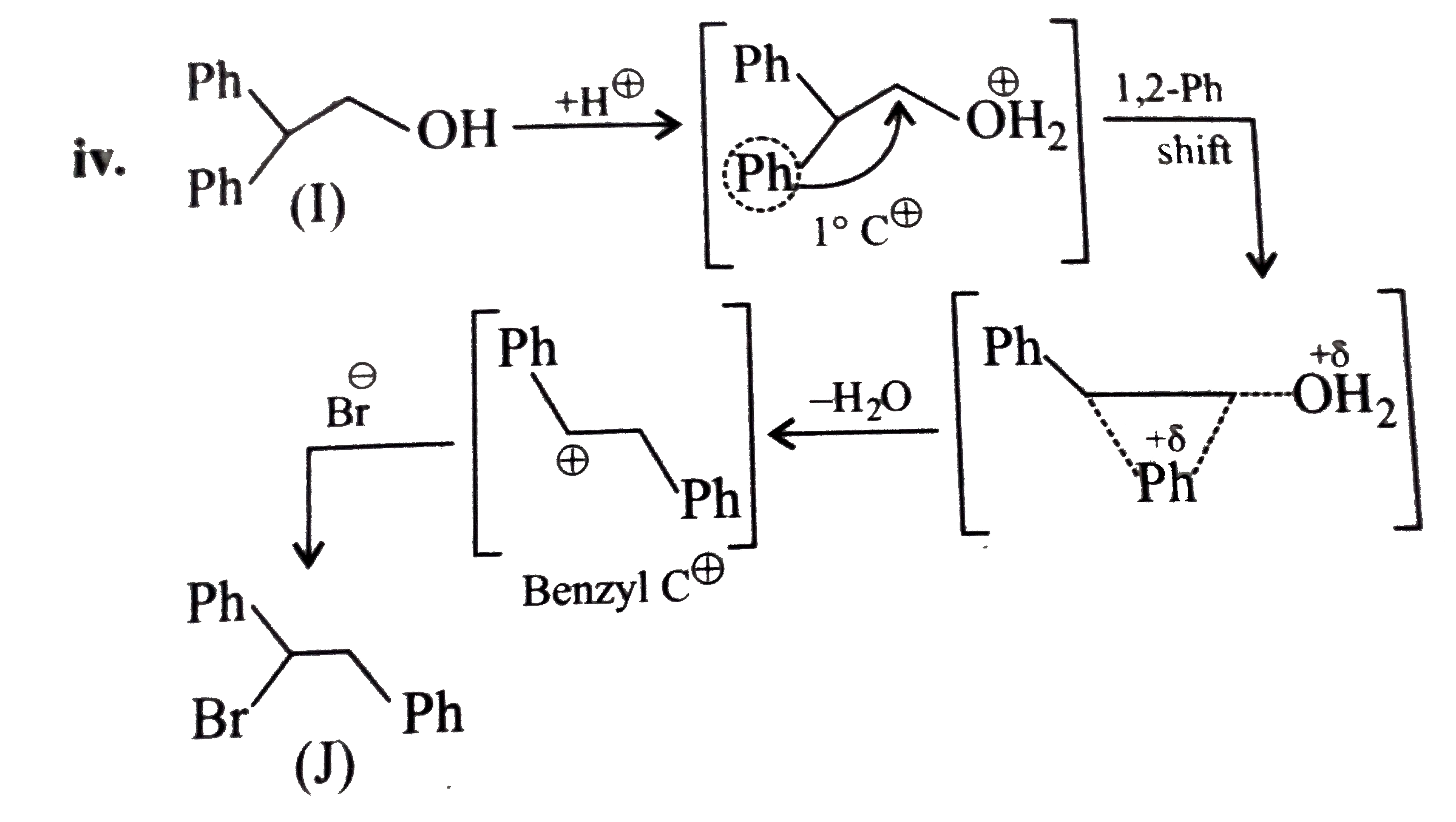
View SolutionWrite the balanced chemical equations for the following reactions :
(i) Manganese dioxide and concentrated hydrochloric acid.
(ii) Sodium thiosulphate and iodine.
(iii) Copper and dilute nitric acid.
(iv) Sulphur dioxide and hydrogen sulphide.
View SolutionName the acid and base from which the following salts are formed.
(a) Sodium chloride
(b) Potassium sulphate
(c) Calcium nitrate
View SolutionThe major product formed upon addition of 1 mole of HBr in the following reaction is :

View Solution