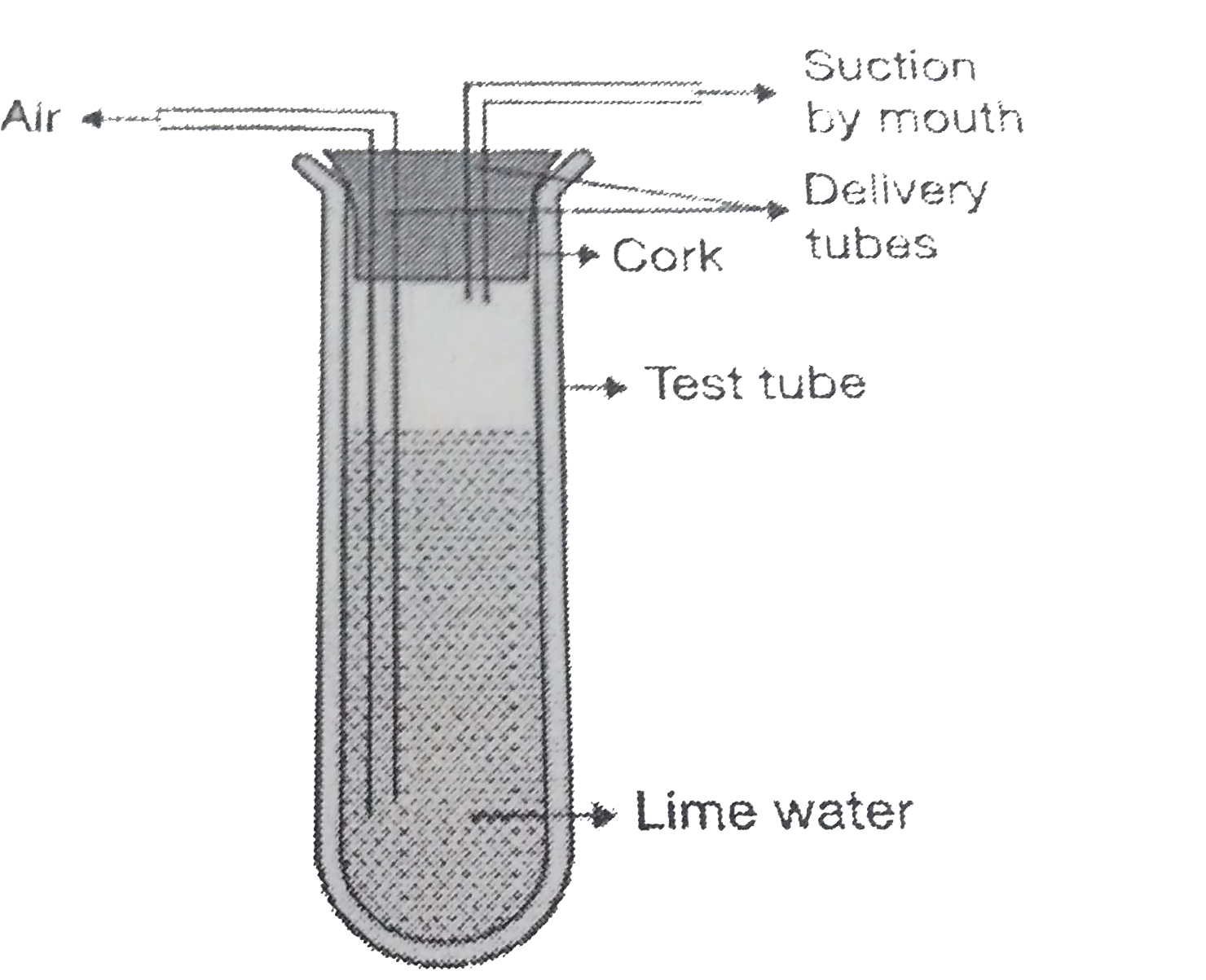
(a) Write the chemical name of the substance taken in the test tube.
(b) What is the observation ? Give reasons.
( c) The air present in the test tube as shown in the diagram is sucked out . why ?
(a) Lime water that is the solution of calcium hydroxide is taken in the test tube.
(b) Lime water turn slighty turbid due to the carbon dioxide present in air.
(c ) The air present in the test tube is sucked in order to create low pressure in the test tube. Due to low pressure , air rusher through the lime water.
Similar Questions
Knowledge Check
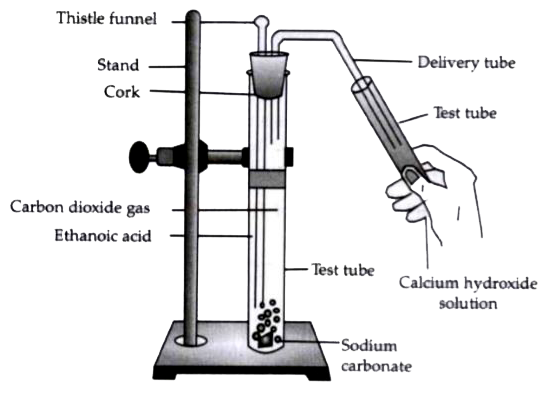
Name the gas released in test tube B.AOxygenBCarbon dioxideCHydrogenDNitrogenA student while heating solid lead nitrate taken in a test tube would observe :
Awhite residue of PbO2Bgreen residue of NO2Cyellow residue of PbODbrown residue of NOAn iron nail is dipped in the solution of copper sulphate for about 30 minture, state the change in colour observed. Give the reason for the change.
Azinc sulphateBcopper sulphateCiron sulphateDaluminium sulphate.
(a) Identify the test tube(s) in which a chemical change take place.
(b) Does the molecular composition remain the same in all the test tubes?
View SolutionA student had taken solutions of KI, H2S,C2H5OH and NaBr in four separate test tube but he forgot to label them. By taking a mall sample of the solution from each of the four test tubes, suggest a method by which he can find out the solution in each test tube by using the same reagent in each of the test tubes. Give the reaction in each case.
View Solution(a) Identify the test tube(s) in which a chemical change take place.
(b) Does the molecular composition remain the same in all the test tubes?

View SolutionArrange the following steps in proper sequence to determine the relative density of liquid by using a constant immersion hydrometer.
(a) The test tube is made to float in the given liquid.
(b) The weight of the test tube with lead shots in air is determined (W1).
( c) The depth of the test tube immersed in water (h) is determined.
(d) The test tube is made to float in water.
(e ) Lead shots are added or removed from the test tube such that the test tube is immersed to the same depth (h) in the given liquid.
(f) The relative density of the liquid is measured as W1W1.
(g) The test tube is taken out from the given liquid and its weight (W2) is measured.
View Solution2 mL of ethanoic acid was taken in each of the three test tubes A, B and C. To these test tubes 2 mL, 4 mL and 8 mL of water wad added respectively. Which test tube will give a clear solution?
View Solution
