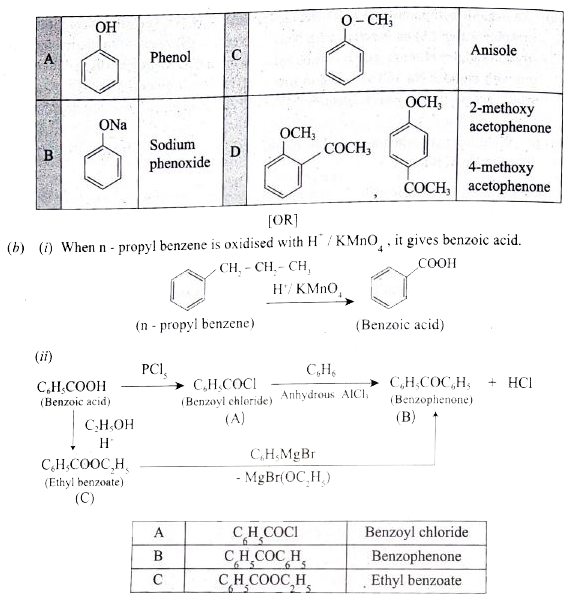
(a) An organic compound (A) of molecular formula C6H6O gives white preclpitate with bromine water. (A) on reaction with NaOH gives (B). (B) reacts with methyl lodlde in presence of dry ether gives (C) of molecular formula C7H8O which will not liberate H2 gas with metallic Na (C) reaction with acetyl chloride gives (D) and (E) of formula which are position isomers. Identify A,B,C,D &E and explain the reaction
(b ) (i) What happens when n-propyl benzene is oxidised using H+/KMnO4
(ii) Identify A,B, andC
 Solution in Tamil
Solution in Tamil
a 1. An organic compound gives white precipitate with bromic water means it must be a phenol from the molecular formula it is identified as C6H5OH
2. Phenol on reaction with NaOH gives (b) as sodium phenoxide C6H5ONa
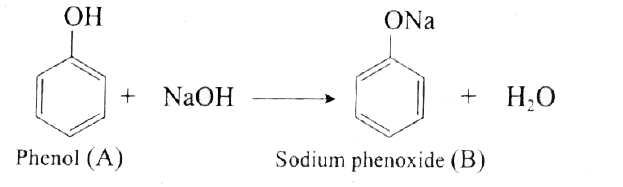
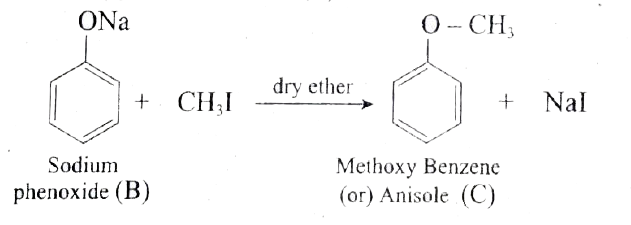
3. Sodium phenxide on reaction with methyl iodide in the pressure of dry ether undergo Williamsons synthesis and gives Anisole as (C )
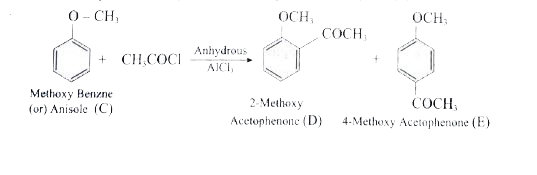
4. Anisole on reaction with acetly chloride undergoes Friedel Craft acetylation yield o-methoxy acetophenone and p- methoxy acetphenone as (d ) and (E )
Similar Questions
An organic compound (A) of molecular formula C_(7)H_(6)O is called as oil of bitter almonds. (A) on oxidation gives (B) of molecular formula C_(7)H_(6)O_(2) which gives brisk effervescence with aqueous alcoholic KCN, compound ( C) is formed. Identify A, B and C and write the equations.
View SolutionCompound A of molecular formula C_(7)H_(6)O reduces Tollen's reagent when A reacts with 50% NaOH gives compound B of molecular formula C_(7)H_(8)O and C of molecular formula C_(7)H_(5)O_(2)Na Compound C on tretatment with dil HCL gives compound D of molecular formula C_(7)H_(6)O_(2) . when D is heated with sodalime gives compound E. Identify A, B, C,D & E. write the corresponding equations .
View SolutionAn organic compound (A) of molecular formula C7H8O on oxidation with alkaline KMnO4 gives (B) of formula C7H6O. (B) on reaction with Cl_(2) in the presence of catalyst FeCl3 gives ( C ) of formula C7H5OCl. .(B)onreactionwithCl_(2)∈the|e|nceofcatalystgivesC_(7)H_(5)OCl. Identify A, B, C, D and explain the reaction involved.
View Solution(b) A aromatic hydrocarbon (A) of molecular formula C_(6)H_(6) reacts with Conc. HNO_(3) and Conc. H_(2)SO_(4) gives (B) of formula C_(6)H_(5)O_(2)N . (B) on reaction with Sn//HCl gives (C ) of formula C_(6)H_(7)N which answers carbylamine reaction. (C ) on treatment with chloroform and alkali gives (D ) of formula C_(7)H_(5)N . Identify A, B, C, D and explain the reactions involved.
View SolutionAn organic compound (A) of molecular formula C6H7N on reaction with sodium nitrite and hydrochloric acid at 0∘C gives (B) or formula C6H5N2Cl (B) on treatment with cuprous cyanide give (C) of formula C7H5N. (C) on reaction with sodium and ethanol gives (D) of formula C7H9N. (D) on reaction with nitrous acid gives (E) of molecular formula C7H8O. Identify A,B,C,D and E and explain the reactions involved.
View SolutionAn organic compound P has molecular formula C2H4. On reduction, it gives another compound Q with molecular formula C2H6. Q reacts with chlorine in the presence of sunlight to give R having molecular formula C2H5Cl.
Identify P, Q and R.
View SolutionAn organic compound A of molecular formula C_(2)H_(4) , on reduction gives another compound B of molecular formula C_(2) H_(6) .B on reaction with chlorine in the presence of sunlight gives C of molecular formula C_(2)H_(5) CI . The compounds A, B and Care:
View Solution

