Can we use nucleophiles such as NH3,CH3O for the Nucleophilic substitution of alcohols. Solution in Tamil
Increasing order of nucelophilicity,
NH3<−OH⊕<CH3OΘ−
Higher electron density will increase the nucelophilicity.
Negatively chrged species are almost always more nucelophiles than neutral species.
ROΘ has an alkyl group attached, allowing a greater amount of polarizability. This means oxygen.s lone pairs will be more readily available to reach in ROΘ than in OHΘ. Hence CH3O− is the better nucelophile for the nucleophilic substitution of alcohols.
NH3 cannot act as nucelophile for the nucleophilic substitution of alcohols.
Similar Questions
Can we use nucelophiles such as NH3,CH3O− for the Nucleophilic substitution of alcohols
View SolutionIn nucleophilic substitution nucleophile will substitute
View SolutionIn nucleophilic substitution nucleophile will substitute
View SolutionThe decreasing order of nucleophilicity among the nucleophiles is :
(I) CH3−C∣∣O−O−
(II)CH3O−
(III) CN− (IV)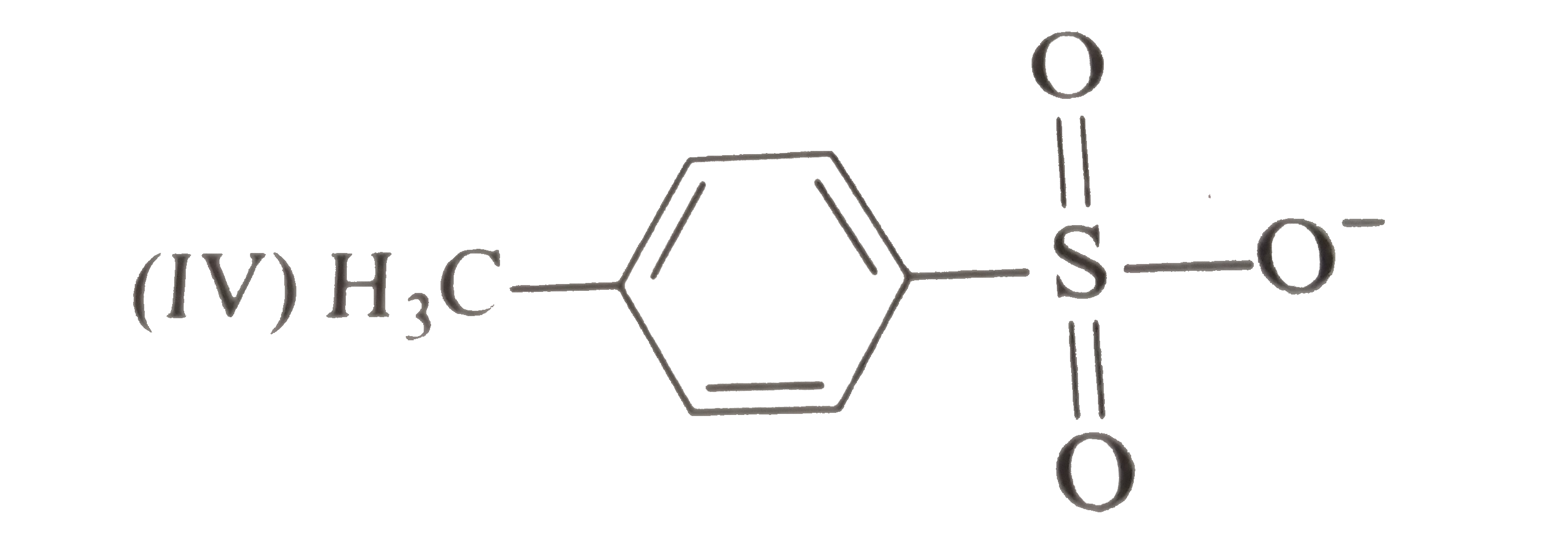
View SolutionHow many of the following nucleophiles are better than NH3 for nucleophilic substitution reaction
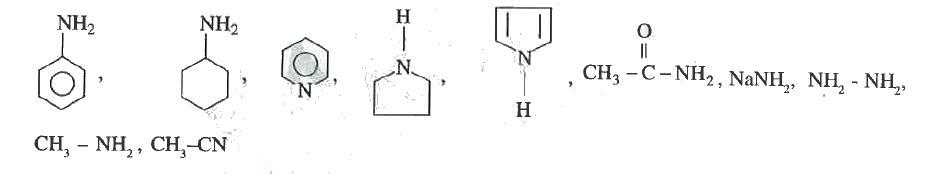
View SolutionWrite the products of nucleophilic substitution reaction of alkyl halide with NH_(3(alc))
View SolutionWhich of the following does not give nucleophilic substitution with alcohol :
View SolutionOutline the preparation of following compounds using a nucleophilic of following compounds using a nucleophilic substitution reaction. CH_(3)-underset(underset(OH)|)CH-CH_(3)
View Solution
