(i) How would you calculate the solubility of sparingly soluble salt using Kohlrausch's law ?
(ii) Formic acid act as reducing agent. Prove this statement. Solution in Tamil
(i) Substances like AgCl,PbSO4 are sparingly soluble in water. The solubility product can be determined using conductivity experimentss.
2. Let us consier AgCl as an example
AgCl(s)↔Ag++Cl−
KSP=[Ag+][Cl−]
3. Let the concentration of [Ag+] be .C. mol L−1
If [Ag+] = C, then [Cl−] is also equal to C mol L−1
∴Ksp=C.C
Ksp=C2
4. The relationship between molar conductance and equivalent conductance is
A∘=K×10−3C(molL−1)(or)C=K×10−3A∘
Substitute the concentration value in the relation Ksp=C2
Ksp=[K×10−3A∘]2
(ii) 1. Formic acid contains both an aldehyde as well as an acid group. Hence, like other aldehydes, formic acid can easily be oxidised and therefore acts as a strong reducing agent.
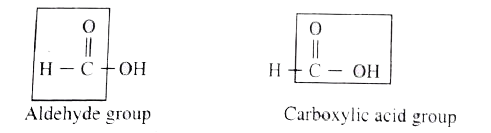
2. Formic acid reduces Tollen.s reagent (ammonical silver nitrate solution) to metallic silver.
HCOO−+2Ag++3OH−(Tollen.s reagent)→2Ag+CO2−3(Silver mirror)+2H2O
3. Formic acid reduces Fehling.s solution. It reduces blue coloured cupric ions to red coloured cuprous ions.
HCOO−+2Cu2++5OH−(Fehlings solution)(Blue)→Cu2O↓Red precipitate+CO2−3+3H2O
Similar Questions
Solubility Product in Sparingly Soluble Salts
View SolutionSTATEMENT-1: Sparingly soluble salts AB and XY2 with the same solubility product, will have different solubility.
STATEMENT-2: Solubiluty of sparingly soluble salt depend upon solubility product.
View SolutionDiscuss the applications of calculation of solubility product of a sparingly soluble salt.
View SolutionWhich of the following is sparingly soluble salt ?
View SolutionAgCl is a sparingly soluble salt and ……..
View SolutionWhat is meant by sparingly soluble salt?
View SolutionCalculation OF degree OF dissociation OF weak electrolyte||Calculation OF solubility product OF sparingly soluble salt
View SolutionThis question has statement I and Statement II. Of the four choices given after the Statements, choose the one that best describes the two Statements.
Statement-I: Sparingly soluble salts AB and XY2 with the same solubility product, will have different solubility.
Statement-II: Solubility of sparingly soluble salt depend upon solubility product.
View Solution

