(a) (i) A solution of [Co(NH3)4I2]Cl2 when treated with AgNO3 gives a white precipitate. What should be the formula of isomer of the dissolved complex that gives yellow precipitate with AgNO3. What are the above isomers called?
(ii) Write the following in the complex [Cr(en)3][CrF6]
(i) Type of complex (ii) Ligands (iii) central metal
(iv) Oxidation state of central metal (v) IUPAC nameSolution in Tamil
(a) (i) 1 A solution of [Co(NH3)4I2]Cl when treated with AgNO3 gives a white precipitate. Because Cl− ion is counter ion.
2. Formula of isomer of the dissolved complex that give yellow precipitate with AgNO3 is [Co(NH3)4Cl]I because I^(ө) is counter ion
3. [Co(NH3)4I2]Cland[Co(NH3)4ClI] I both are ionisation isomers
(ii) 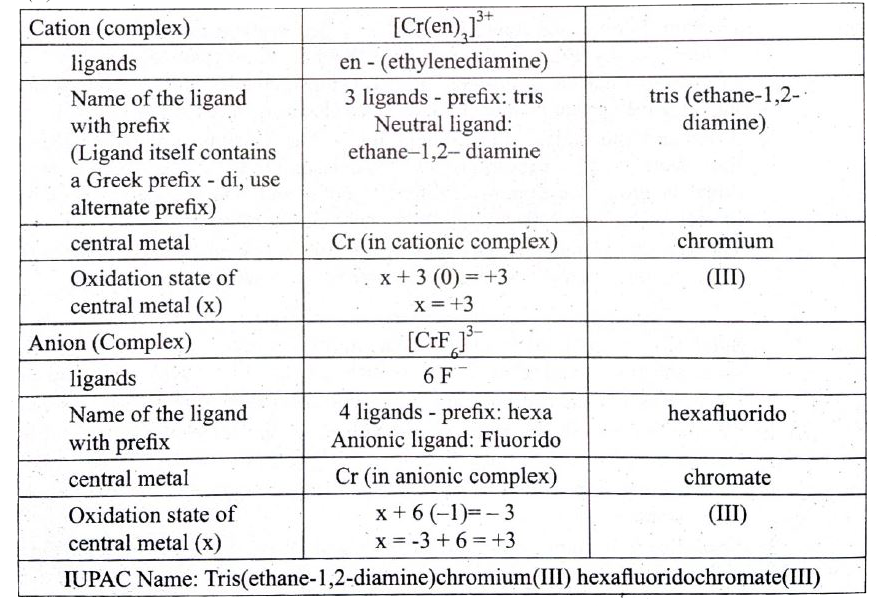
Similar Questions
The question consist of two statements each, printed as Assertion and Reason. While answering these questions you are required to choose any one of the following four responses:
Assertion: The complex [Co(NH3)3Cl3] does not give precipitate with AgNO3.
Reason: The given complex does not contain counter ions.
View SolutionA solution of [Co(NH3)4I2]Cl when treated with AgNO3 gives a white precipitate What should be the formula of isomer of the dissolved complex that gives yellow precipitate with AgNO3 What are the above isomers called?
View SolutionWrite IUPAC name of the complex [Cr(NH3)4Cl2]+. Draw structures of geometrical isomers for this complex.
View SolutionAn isomer of the complex Co(en)_(2)(H_(2)O)IC l_(2) , on reaction with concentrated H_(2)SO_(4) , it suffers loss in weight and on reaction with AgNO_(3) solution gives a yellow precipitated which is isoluble in NH_(3) solution. Q. Total number of space isomers of the formula of the above complex is:
View Solutionनिम्न में से कौन - सा संकुल जलीय में AgNO3 के साथ सफ़ेद अवक्षेप देगा ?
View SolutionA solution of [Co(NH_(3))_(4)I_(4)]Cl when treated with AgNO_(3) gives a white precipitate.What should be the formula of isomer of the dissolved complex that gives yellow precipitate with AgNO_(3) . What are the above isomers called ?
View SolutionIdentify and write the following in the complex [CO(NH_(3))_(4)Cl_(2)]Cl (i) Cation (ii) Ligands (iii) Name of the ligand (iv) central metal (v) Oxidation state of central metal (vi) Anion (vii) IUPAC name
View Solution
