Deduce the oxidation number of oxygen in hypofluorous acid −HOF.
Text Solution
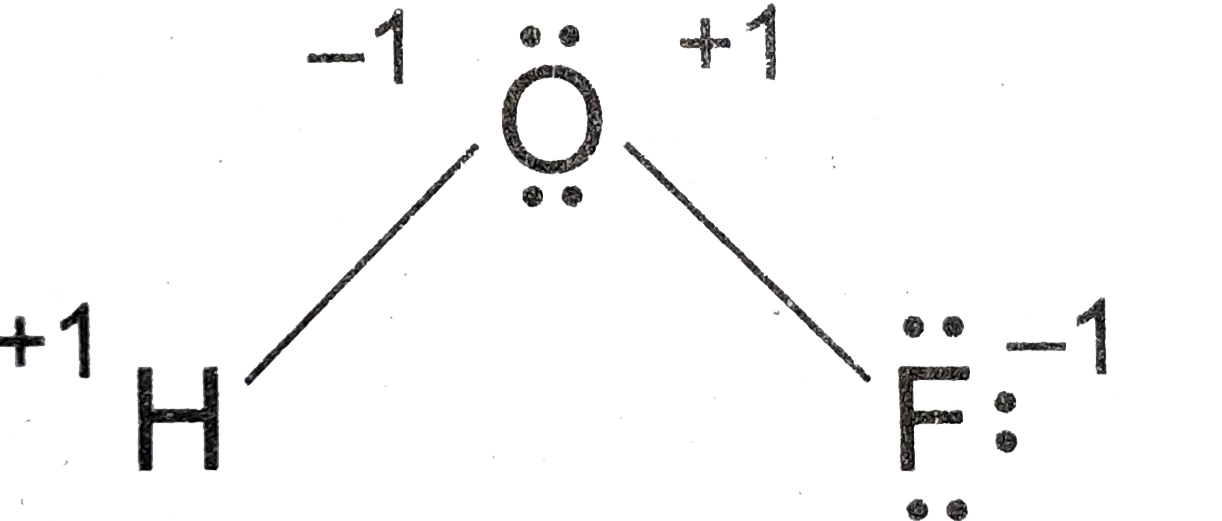
In case of O−F bond is HOF, fluorine is most electronegative element. So its oxidation number is −1. Thereby oxidation number of O is +1. Similarly in case of O−H bond is HOF. O is highly electronegative than H. So its oxidation number is −1 and oxidation number of H is +1. So, Net oxidation of oxygen is −1+1=0.
|
Similar Questions
Number of Oxygen atoms required for complete oxidation of pyruvic acid is
View SolutionOF2 में ऑक्सीजन की ऑक्सीकरण संख्या है
View SolutionDeduce the oxidation number of S in the following species :
SO2
View SolutionOxidation number of oxygen is superoxide is
View SolutionThe percentages of oxygen in two oxides of a metal are 22.2 and 30 respectively. The formula of the second oxide is M_2O_3 . Deduce the formula of first.
View SolutionKO2 में ऑक्सीजन की ऑक्सीकरण संख्या है
View SolutionNumber of oxygen atoms required for complete oxidation of pyruvic acid is
View SolutionNumber of Oxygen atoms required for complete oxidation of pyruvic acid is
View Solution

