[Ni(CN)4]2− is diamagnetic , while [NiCl4]2− is paramagentic, explain using crystal field theory.
(a)[Ni(CN)4]2−
Ni=3d84S2
Ni2+=3d8
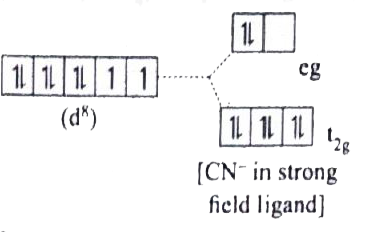
Nature of the complex-Low spin (Spin paired)
Ligand filled electronic configuration of central metal ion, t62ge2g
Magnetic property : No unpaired electron (CN− is strong filled ligand ), hence it is dimagnetic.
Magnetic moment : μs=0
(b)=[NiCl4]2−
Ni=3d84S2
Ni2+=3d8
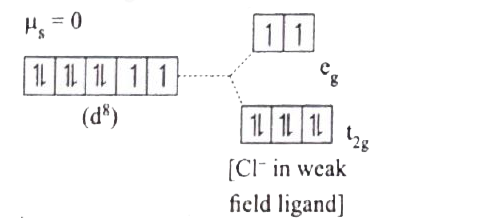
Naute of the complex,-high spin
Ligand filled electronic configuration of central metal ion , t62ge2g
Magnetic property : Two unpaired electron (Cl− is weak field ligand). Hence it is paramagnetic
Magnetic moment : μs=√2(2+2)
=√8=2.83BM
Similar Questions
Why is [NiCl4]2− paramagnetic while [Ni(CN4)]2− is diamagnetic ? (At. No. of Ni = 28)
View SolutionExplain on the basis of valence bond theory that [Ni(CN)4]2− ion with square planar structure is diamagnetic and the [NiCl4]2− ion with tetrahedral geometry is paramagnetic.
View Solutionसंयोजकता आबंध सिद्धांत के आधार पर समझाइये कि वर्ग समतलीय संरचना वाला [Ni(CN)4]2− आयन प्रतिचुंबकिय है तथा चतुष्फलकीय ज्यामिति वाला [NiCl4]2− आयन अनुचुंबकिय है।
View Solution[Ni(CN)4]2− प्रतिचुम्बकिये है जबकि [NiCl4]2− अनुचुम्बकिये है, क्यों?
View Solution[Ni(CN)4]2− is diamagnetic ,while [NiCl4]2− is paramagnetic ,explain using crystal field theory.
View SolutionExplain on the basis of valence bondy theory that [Ni(CN)4]2− ion with square planar structure is diamagnetic and the [NiCl4]2− ion with tetrahedral geometry is paramagnetic.
View Solution[Ni(CN)4]2− diamagnetic, whereas [NiCl4]2− is paramagnetic. Explain.
View Solution

