Explain optical isomerism in coordination comounds with an example.
Text Solution
(i) Coordination compounds which possess chairality exhibit optical isomerism similar to organic compounds.
(ii) The pair of two optically acitve isomers which are mirror images of each other are called enantiomers.
(iii) Their solutions rotate the plane of the plane polarised light either clockwise or anticlockwise and the corresponding isomers are called d (dextrorotatry) and I (levelortatory) forms respectively.
(iv) The octahedral complexes of type
[M(xx)3]n±, [Mxx)2AB]n± and [M(xx)2B2]n± exhibit optical isomerism.
Examples :
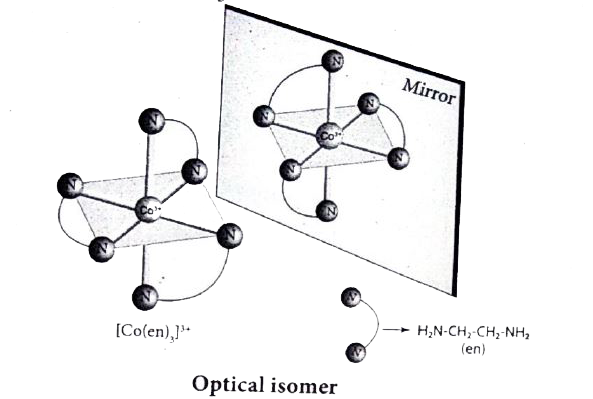
(i) The optical isomers of [Co(en)3]3+ are shown below.
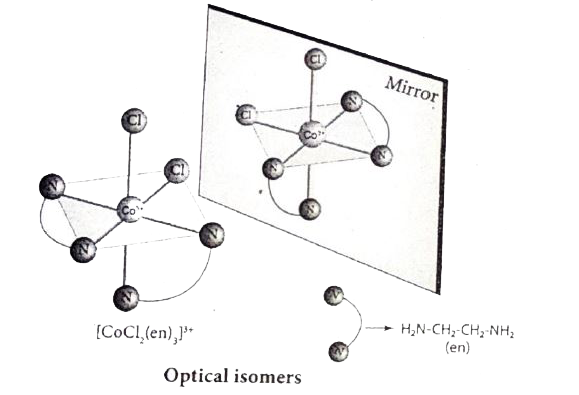
(ii) The coordination complex [CoCl2(en)2]+ has three isomers, two optically active cis forms and one optically inactive trans form. These sturctures are shown below.
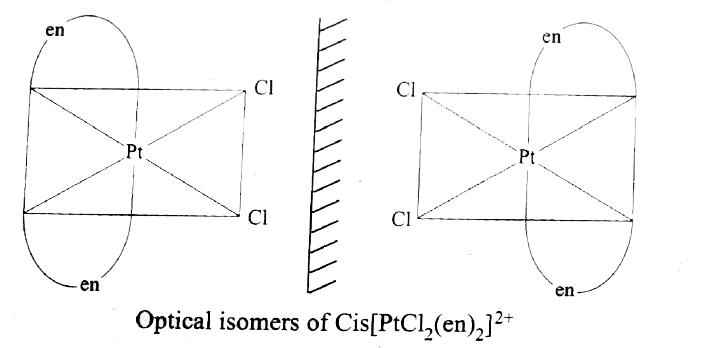
(iii) In a coordination compound of type [PtCl2(en)2]2+, two geometrical isomers are possible . They are cis and trans. Among these two isomers cis isomer shows optically active isomerism because the whole molecule is asymmetric.
|
Similar Questions
Knowledge Check
An example of coordination isomerism is
A[Cr(H2O)6]Cl3and[Cr(H2O)5Cl]Cl2,H2OB[Co(NH3)6][Cr(CN)6]and[Cr(NH3)6][Co(CN)6]C[Pt(NH3)4Cl2]and[Pt(NH3)2Cl4]D[Co(NH3)5Br]SO4and[Co(NH3)5SO4]Br
What is meant by functional isomerism ? Explain with an example.
View SolutionExplain geometrical isomerism with the help of suitable example.
View SolutionWhat is isomerism? Give a precise definition and explain with examples.
View SolutionAn example of coordination isomerism is
View SolutionWhat are coordination isomers and optical isomers. Explain with atleast one example.
View SolutionExplain the terms optically rarer medium and optically denser medium with examples.
View SolutionAn example of coordination isomerism is
View Solution

