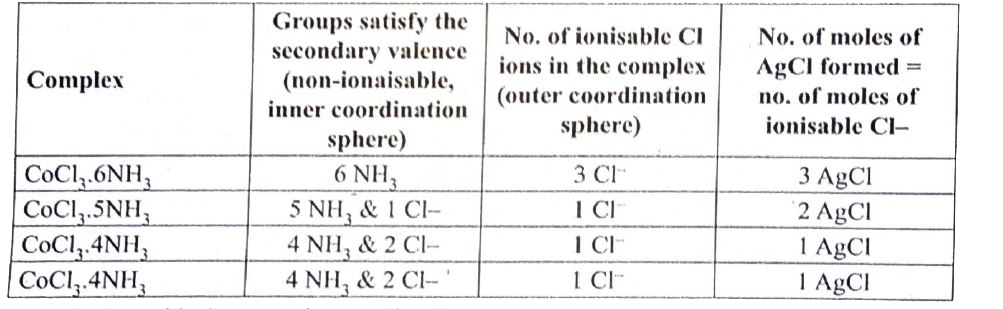
Explain Werner's postulate using COCl3.6NH3.
Text Solution
|
Topper's Solved these Questions
- COORDINATION CHEMISTRYBOOK - FULL MARKSCHAPTER - COORDINATION CHEMISTRYEXERCISE - Additional Question(5 Marks Question)
26 Videos
- COORDINATION CHEMISTRYBOOK - FULL MARKSCHAPTER - COORDINATION CHEMISTRYEXERCISE - Additional Question(2 Marks Question)
23 Videos
- CHEMISTRY IN EVERYDAY LIFE BOOK - FULL MARKSCHAPTER - CHEMISTRY IN EVERYDAY LIFE EXERCISE - ADDITIONAL QUESTION ( 5 Mark Questions )
2 Videos
- ELECTRO CHEMISTRYBOOK - FULL MARKSCHAPTER - ELECTRO CHEMISTRYEXERCISE - ADDITIONAL QUESTIONS -5- MARK QUESTIONS
15 Videos
Similar Questions
Knowledge Check
In the compound CoCl3.5NH3 :
Aall the cl show primary valency (PV) onlyBtwo cl show (PV) and one Cl show secondary valency (SV)Ctwo Cl show (PV) and one Cl (PV) as well as (SV)Dall the Cl show (SV)In the compound CoCl3.5NH3 :
Aall the cl show primary valency (PV) onlyBtwo cl show (PV) and one Cl secondary valency (SV)Ctwo Cl show (PV) and one Cl (PV) as well as (SV)Dall the Cl show (SV)In the complex CoCl3.4NH3
ACo-ordiantion entity is [Co(NH3)4.Cl2]+BCounter ion is Cl−CBoth are correctDNone is correct.
The colour of CoCl3⋅5NH3⋅H2O is
View SolutionThe colour of CoCl3.5NH3.H2O is
View SolutionNumber of chlorides satisifying secondary valency in CoCl34NH3
View SolutionThe colour of CoCl3⋅5NH3⋅H2O is
View SolutionGiven the molecular of the hexacoordinated complexes is :
(1)CoCl3.6NH3 (2)CoCl3.5NH3 (3)CoCl3.4NH3
If the number of co-ordinated NH3 molecules in (1),(2) and (3) respectively are (6),(5) and (4) , the primary valency in (1),(2) and (3) are :
View Solution