Identify the conjugate acid base pair for the following reaction in aqueous solution
HS−(aq)+HF↔F−(aq)+H2S(aq)
(ii) HPO2−4+SO2−3↔PO3−4+HSO−3
(iii) NH+4+CO2−3↔NH3+HCO−3

HFandF−,HS−andH2S are two conjugate acid-base pairs.
F− is the conjugate base of the acid HF (or) HF is the conjugate acid of F−
H2S is the conjugate acid of HS− (or) HS− is the conjugate base of H2S.
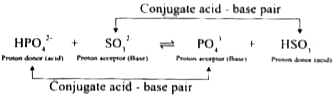
HPO2−4andPO3−4,SO2−3andHSO−3 are two conjugate acid-base pairs.
PO3−4 is the conjugate base of the acid HPO2−4 (or) HPO2−4 is the conjugate acid of PO3−4
HSO−3 is the conjugate acid of SO2−3 (or) SO2−3 is the conjugate base of HSO−3

NH+4andNH3,CO2−3andHCO−3 are two conjugate acid -base pairs.
HCO−3 is the conjugate of acid CO2−3 (or) CO2−3 is the conjugate bases of HCO−3.
NH4 is the conjugate base of NH+4 (or) NH+4 is the conjugate acid of NH3
Similar Questions
Knowledge Check
Which of the following comments about the following equilibrium reaction is the most correct ?
H2SO4(aq)+NH3(aq)<⇒HSO−(aq)4+NH+4(aq)AThe equilibrium lies mostly to the rightBThe equilibrium lies mostly to the leftCThe equilibrium is exactly in between where the concentration of each of the reactants is exactly equal to the concentration of each of the productsDThe equilibrium depends on the temperature of the equilibrium reaction
What is the Lewis acid and base in the following reaction?
2NH3(aq)+Ag+(aq)→[Ag(NH3)2]+(aq).
View SolutionWhat is the Lewis acid and base in the following reaction?
2NH3(aq)+Ag+(aq)→[Ag(NH3)2]+(aq).
View SolutionWrite the equilibrium constant expressions for the following reactions.
NH3(aq)+H2O(l)⇔NH+4(aq)+OH−(aq)
View SolutionWhat do you understand by conjugate pairs of acid and base? Sort out the conjugate pairs in the following reactions.
NH+4(aq)+S2−(aq)⇔HS−(aq)+NH3(aq)
View SolutionClassify the following as acid or base and also mention the concept on the basis of which these are so.
(i) HClaq (ii) Na2CO3(aq)
(iii) H2O (iv) NH4
View SolutionWhat do you understand by conjugate pairs of acid and base? Sort out the conjugate pairs in the following reactions.
H2O(l)+CO2−3(aq)⇔HCO−3(aq)+OH−(aq)
View SolutionOn adding NH4HS in following equilibrium NH4HS(s) ↔ NH3(g)+H2S(g)
View Solution

