Define solubility product.
Text Solution
Solubility product: It is defined as the product of the molar concentration of the constituent ions, each raised to the power of its stoichiometric co-effcient in a balanced equilibrium equation.
XmYn(s)↔H2OmXn+(aq)+nYm−(aq)
Ksp↔[Xn+]m[Ym−]n
|
Similar Questions
Knowledge Check
Solubility product is
AThe ionic product of an electrolyte in its saturated solutionBThe product of the solubilities of the ions of the electrolyteCThe product of solubilities of the saltsDThe product of the concentration of the ionsAssertion : A ionic product is used for any types of electrolytes whereas solubility product is applicable only to sparingly soluble salts.
Reason : ionic product is defined at any stage of the reaction whereas solubility product is only applicable to the saturation stageAIf both assertion and reason are true and reason is the correct explanation of the assertionBIf both assertion and reason are true but reason is not the correct explanation of the assertion.CIf assertion is true but reason is false.DIf the assertion and reason both are false.Statement-1: Sparingly soluble salts AB and XY2 with the same solubility product, will have different solubility.
Statement 2: Solubility of sparingly soluble salts depends upon solubility product.AIf both the statements are true and statement -2 is the correct explanation of statement-IBIf both the statements are true but statement-2 is not the correct explanation of statement-ICIf statement-I is true and statement-2 is falseDIf statement-I is false and statement-2 is true.
The stoichiometre and solubility product oFIGURE a salt with the solubility curve given below is respectively.
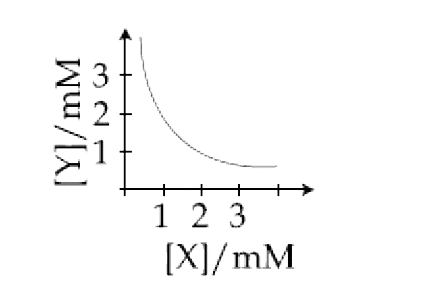
View SolutionWrite the solubility product of sparingly soluble salt Bi2S3
View SolutionIf s and S are respectiveluy solubility and solubility product of a sparingly soluble binary electrolyte , then :
View SolutionWhat will be the solubility product of Ca(OH)2 if its solubility is √3.
View SolutionIf s and S are respectiveluy solubility and solubility product of a sparingly soluble binary electrolyte , then :
View Solution

