Write a balanced equation for the dissociation of the following in water and identify the conjugate acid-base pairs.
NH+4
(ii) H2SO4
(iii) CH3COOH

NH+4andNH3,H2OandH3O+ are two conjugate acid base pairs.
(ii) 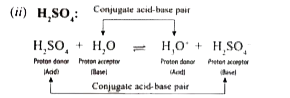
H2SO4andHSO−4,H2OandH3O+ are two conjugate acid base pairs.
(iii) 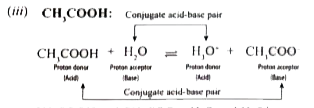
CH3COOHandCH3COO−,H2OandH3O+ are two conjugate acid base pairs.
Topper's Solved these Questions
- IONIC EQUILIBRIUMBOOK - FULL MARKSCHAPTER - IONIC EQUILIBRIUMEXERCISE - ADDITIONAL QUESTIONS (CHOOSE THE BEST ANSWER)
51 Videos
- IONIC EQUILIBRIUMBOOK - FULL MARKSCHAPTER - IONIC EQUILIBRIUMEXERCISE - ADDITIONAL QUESTIONS (FIND THE ODD ONE OUT AND GIVE THE REASONS )
12 Videos
- IONIC EQUILIBRIUMBOOK - FULL MARKSCHAPTER - IONIC EQUILIBRIUMEXERCISE - TEXTBOOK EVALUATION (ANSWER THE FOLLOWING QUESTION:)
27 Videos
- HYDROXY COMPOUNDS AND ETHERSBOOK - FULL MARKSCHAPTER - HYDROXY COMPOUNDS AND ETHERSEXERCISE - ADDITIONAL QUESTIONS (Answer the following)
115 Videos
- METALLURGY BOOK - FULL MARKSCHAPTER - METALLURGY EXERCISE - Additional questions - ( 5 MARK QUESTIONS )-Answer the following questions.
8 Videos
Similar Questions
Write the proton transfer equilibria for the following acids in aqueous solution and identify conjugate acid base pairs in each :
(i)HCN , (ii)CH3COOH
View SolutionWrite the reaction of benzyl magnesium obmide with CH3OD and also identify the conjugate acid-base pairs.
View SolutionWrite the reaction of benzyl magnesium obmide with CH3OD and also identify the conjugate acid-base pairs.
View SolutionGive the conjugate bases of
(i) H2O (ii) HNO3 (iii) NH+4 (iv) HCO−13
View SolutionGive the conjugate bases of
(i) H2O (ii) HNO3 (iii) NH+4 (iv) HCO−13
View SolutionWrite the formula of conjugate acids of :
(i) C6H5OH , (ii) NH2NH2 , (iii) HCO−3
View SolutionWrite conjugate acid for the following :
(i) C2H5NH2 (ii) CH3COOH
View SolutionWrite down the conjugate base of the following: (i) NH+4,(ii) HCOOH (iii) H3O+
View SolutionWrite the conjugate base of CH3COOH+H2O.
View SolutionWrite the conjugate acid and the conjugate base of the following:
NH3
View Solution

