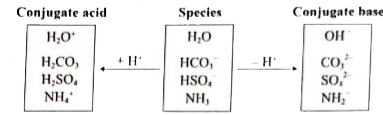
The species H2O,HCO−3,HSO−4andNH3 can act both as Bronsted acid and base. For each case, given the corresponding conjugate acid and base.
Topper's Solved these Questions
- IONIC EQUILIBRIUMBook:FULL MARKSChapter:IONIC EQUILIBRIUMExercise:ADDITIONAL QUESTIONS (3 MARK QUESTION)
Explore 20 Videos
- IONIC EQUILIBRIUMBook:FULL MARKSChapter:IONIC EQUILIBRIUMExercise:ADDITIONAL QUESTIONS (5 MARK QUESTION)
Explore 15 Videos
- IONIC EQUILIBRIUMBook:FULL MARKSChapter:IONIC EQUILIBRIUMExercise:ADDITIONAL QUESTIONS FIND OUT THE INCORRECT PAIRS
Explore 1 Video
- HYDROXY COMPOUNDS AND ETHERSBook:FULL MARKSChapter:HYDROXY COMPOUNDS AND ETHERSExercise:ADDITIONAL QUESTIONS (Answer the following)
Explore 115 Videos
- METALLURGY Book:FULL MARKSChapter:METALLURGY Exercise:Additional questions - ( 5 MARK QUESTIONS )-Answer the following questions.
Explore 8 Videos
Similar Questions
The species H2O,HCO−3,HSO−4andNH3 and act both as Bronsted acid and base. For each case, give the correponding conjugate acid and base.
View SolutionThe species: H2O,HCOΘ3,HSOΘ4 and NH3 can act both as Bronsted acids and bases. For each case give the corresponding conjugate acid and base.
View Solution
Knowledge Check
Species acting both as Bronsted acid and base is
AHSO−4BH2BO−3CNH3DOH−Species acting both as Bronsted acid base is
AHSO−4BNa2CO3CNH3DOH−
The species: H2O,HCOΘ3,HSOΘ4 and NH3 can act both as Bronsted acids and bases. For each case give the corresponding conjugate acid and base.
View SolutionThe species: H2O,HCOΘ3,HSOΘ4 and NH3 can act both as Bronsted acids and bases. For each case give the corresponding conjugate acid and base.
View SolutionThe species: H2O,HCOΘ3,HSOΘ4 and NH3 can act both as Bronsted acids and bases. For each case give the corresponding conjugate acid and base.
View SolutionThe species H2O,HCOΘ3,HSOΘ4,NH3 can act both as Bronsted acids and bases. For each case write the corresponding conjugate acid.
View SolutionThe species H2O,HCOΘ3,HSOΘ4,NH3 can act both as Brddotosted acis and bases. For each case give the corresponding conjugate acid and conjugate base.
View SolutionWrite four species which act both as Bronsted acid as well as base. Write their corresponding conjugate acids and bases.
View Solution