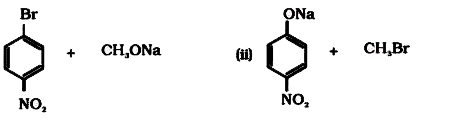
1. Which of the following reaction will give 1-methoxy-4-nitrobenzene.
(a) 4-nitro-1-bromobenzene + sodium methoxide.
(b) 4-nitrosodium phenoxide + bromomethane
(a) Set - (a) :
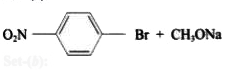
(b) Set - (b) :
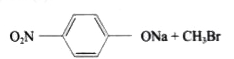
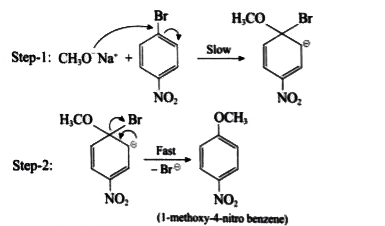
Chemically both sets equally possible. In Set-1, the Br-atom is activated by electron withdrawing effect of −NO2 group. Therefore nucleophilic attack by CH3ONa followed by elimination of NaBr gives the desired ether.
In set-2, nucleophilic attack by 4-nitrosodium phenoxide ion on methyl bromide gives the desiredether
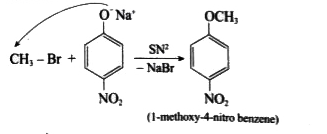
Since alkyl halides (CH3Br) are more reactive than aryl halides in nucleophilic substitution reactions, therefore set-2 reactants are preferTed.
Similar Questions
1 Which of the following is an appropriate set of reactants for the preparation of 1-methoxy-4-nitrobenzene and why?
View SolutionWhich of the following set of reactants will give 1-methoxy-4-nitrobenzene.
View SolutionWhich of the following is an appropriate set of reactants for the preparation of 1-methoxy-4 nitrobenzne and why ?
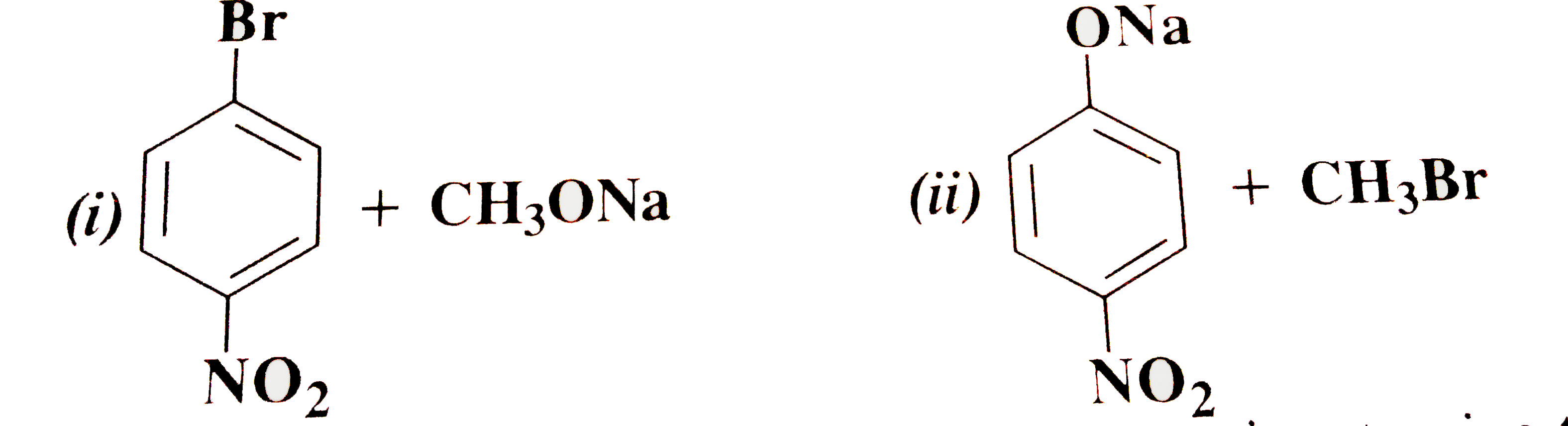
View SolutionWhich is the correct increasing order of boiling points of the following compounds ? 1-bromomethane, 1-bromobutane, 1-chlorobutane, Bromobenzene
View SolutionWhich of the following is an appropriate set of eactants for the preparation of 1-methoxy-4 nitrobenzene. Why?
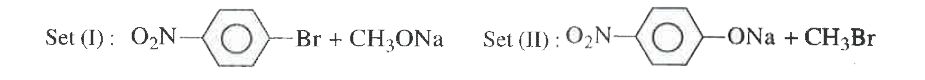
View Solution1-मेथॉक्सी-4-नाइट्रोबेंजीन बनाने के लिए निम्न में से अभिकर्मकों का कौनसा सेट उचित है
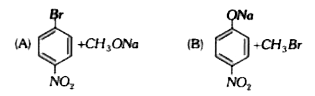
View SolutionWhich of the following is an appropriate set of reactants for the preparation of 1-methoxy-4-nitrobenzene and why?

View Solution

