An ether is more volatile than an alcohol having the same molecular formula. This is due to ……………………. .
dipolar character of ethers
alcohols having resonance structures
inter molecular hydrogen bonding in ethers
inter molecular hydrogen bonding in alcohols
The correct Answer is:D
inter molecular hydrogen bonding in alcohols
Topper's Solved these Questions
- HYDROXY COMPOUNDS AND ETHERSBOOK - FULL MARKSCHAPTER - HYDROXY COMPOUNDS AND ETHERSEXERCISE - ADDITIONAL QUESTIONS (Fill in the blanks)
66 Videos
- HYDROXY COMPOUNDS AND ETHERSBOOK - FULL MARKSCHAPTER - HYDROXY COMPOUNDS AND ETHERSEXERCISE - ADDITIONAL QUESTIONS (Match the following Column-l with Column-ll using the code given below)
17 Videos
- HYDROXY COMPOUNDS AND ETHERSBOOK - FULL MARKSCHAPTER - HYDROXY COMPOUNDS AND ETHERSEXERCISE - Evaluate Yourself
14 Videos
- ELECTRO CHEMISTRYBOOK - FULL MARKSCHAPTER - ELECTRO CHEMISTRYEXERCISE - ADDITIONAL QUESTIONS -5- MARK QUESTIONS
15 Videos
- IONIC EQUILIBRIUMBOOK - FULL MARKSCHAPTER - IONIC EQUILIBRIUMEXERCISE - ADDITIONAL QUESTIONS (5 MARK QUESTION)
15 Videos
Similar Questions
Cholesteryl alcohol commonly known as …………………… is an important component in our ……………… .
View Solution
View SolutionClassify the following alcohols as 1∘,2∘,and3∘ and give their IUPAC names.
(a) CH3−CH2−CH(OH)CH2−Br∣C(CH3)2 (b) (C2H5)3COH
(C ) CH2=C(CI)−CH(OH)CH3 (d)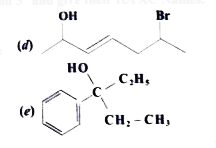
View SolutionHow would you measure the conductivity of ionic solutions?
View SolutionDifferentiate Lewis acids and Lewis bases.
View SolutionAn ether is more volatile then alcohol having same molecular fromula . This is due to :
View SolutionAssertion: Alcohols have higher boiling points than ethers of comparable molecular masses.
Reason: Alcohols and ethers are isomerism in nature.
View Solutionईथर की वाष्पशीलता, समावयवी ऐल्कोहॉल से उच्च होती है, क्यों?
View Solutionसमान अणु भार वाले ऐल्कोहॉलों और ईंथरों में से ईथर अधिक वाष्पशील होता है। इसका कारण है:
View SolutionThe B.P. of alcohols are much higher than the ethers of comparable molecular masses due to
View SolutionBoiling point of alcohol is more than that of ether of corresponding molecular weight , because
View SolutionBoiling point of alcohol is more than corresponding ether. Why ?
View SolutionAssertion: Alcohols have higher boiling points than ethers of comparable molecular masses.
Reason: Alcohols and ethers are isomerism in nature.
View SolutionDimethyl ether and ethanol have same molecular weight, but their volatilities are different-explain.
View Solutionएक ईथर समावयवी ऐल्कोहॉल की तुलना में अधिक वाष्पशील होता है | इसका कारण है-
View Solutionएक समान अणुसूत्र वाले एल्कोहल और ईथर में, ईथर अधिक वाष्पशील हैं इसका कारण है
View Solution

