Explain about the structure of methanol.
(i) In methanol, one of the sps hybridised orbital of oxygen linearly overlap with the sp3 hybridised orbital of carbon to Torm a C-O, σ bond and another sp3 hybridised orbital linearly overlap with ls orbital of hydrogen atom to form a O - Hσ bond.
(ii) 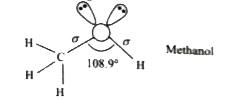
(iii) The remaining two sp3 hybridised orbitals of oxygen are occupied by two lone pairs of electrons. Due to the lone pair - lone pair repulsion, the C-O-H bond angle in methanol is reduced to 108.9∘ from the regular tetrahedral bond angle of 109.5∘.
Similar Questions
Explain about the structure and function of the accessory female organ to which the lower most part of uterus is connected.
View SolutionExplain the structure of graphite.
View SolutionExplain the structure of CIF3.
View SolutionExplain the structure of carbonyl group.
View SolutionExplain the structure of bacteriophage.
View SolutionExplain about the working and structure of ecosystem.
View SolutionDraw the structural formula for
Methanol
View SolutionIdentify and explain about the part of the respiratory system that consists of the clus- ter of structures that resemble grapes for the exchange of gases.
View Solution(a) Write about the various structures of female’s external genitalia.
(b) Explain any two methods of natural contraception.
View Solution(a) Write about the various structures of female’s external genitalia.
(b) Explain any two methods of natural contraception.
View Solution

