Ether are miscible with water. Justify this statement.
Text Solution
(i) Oxygen of ether can also form Hydrogen bond with water and hence they are miscible with water. (ii) Ethers dissolve wide range of polar and non-polar substances.
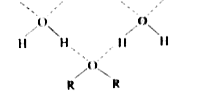
|
Similar Questions
Knowledge Check
Lower alcohols are miscible with water because of the fact that ___________ .
Aalcohols undergo solvolysis with water moleculesBalcohols undergo intermolecular hydrogen bonding with waterCalcohols undergo intramolecular hydrogen bonding with waterDalcohols undergo reduction with water moleculeThe methanol, ethanal and propanone are miscible with water because they form
Avan der Waals' forces with waterBhydrogen bond with waterCdipole-dipole bond with waterDion-dipole bond with water
Ethers are relatively inert. Justify.
View SolutionEthers are relatively inert. Justify.
View SolutionEthers are relatively inert. Justify.
View Solution(a) Explain why cleavage of phenyl ethers with HBr always produces phenol and alkyl bromides and not bromobenzene and alkanols.
(b) Give reasons for the following:
(i) The dipole moment of diethyl ether (1.18D) is lower than that of water (1.84D).
(ii) The C-O-C bond angle in ethers is higher than H-O-H angle in water though oxygen is sp3 hybridized in both the cases.
(iii) Dimethyl ether is completely miscible with water but diethyl ether is soluble in water to a small extent.
View Solution"The war is justified to some extent". Justify the statement.
View SolutionWhy is ether not miscible in water?
View Solution

