Ether bottle should not be kept open. Why?
Text Solution
(i) When ether bottle is kept open, they are exposed to atmospheric oxygen and slowly oxidised to form hydroperoxides and dialkyl peroxides. These are explosive in nature. Such a spontaneous oxidation by atmospheric oxygen is called autooxidation.
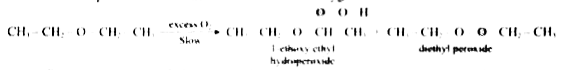
(ii) So to avoid auto oxidation and explosion, ether bottle should be kept closed.
|
Similar Questions
Knowledge Check
Ether bottles should not be kept open in air because-
AEther is an anaestheticBEther forms an explosive peroxideCEther is costlyDEther gets oxidised to ethanolAn open mouthed bottle contains a gas at 60∘C the temperature to which the bottle should be heated so that 1/4 of the mass of the gas may leave is,
A171∘CB250∘CC300∘CD342∘CWhen a bottle of scent is kept open in a corner of a room its odour is felt in all parts of the room. This is due to the phenomenon of:
A(a)evaporationB(b)vaporisationC(c)diffusionD(d)sublimation
During roasting the temperature should be kept below its m.p. Why ?
View SolutionWhy aluminum utensils should not be kept in water overnight.
View SolutionWhy is liquid ammonia bottle first cooled in ice before opening it ?
View SolutionThe bottle of liquid ammonia is generally cooled before opening the seal. Assign reson.
View SolutionWhen soda water bottle is kept open for sometime, it loses the tangy taste. Give reason.
View Solution

