Assertion(A): The boiling point of aldehydes and ketones are much lower those of corresponding alcohols and carboxylic acids.
Reason (R): Alcohols and carboxylic acids possess intermolecular hydrogen bonding and so have high boiling point.
Both A and R are correct and R is the correct explanation of A.
Both A and R are correct but R is not correct explanation of A
A is correct but R is wrong
A is wrong but R is correct
The correct Answer is:B
Topper's Solved these Questions
- CARBONYL COMPOUNDSBOOK - FULL MARKSCHAPTER - CARBONYL COMPOUNDSEXERCISE - ADDITIONAL QUESTIONS (FIND THE ODD OUT AND GIVE THE REASONS)
3 Videos
- CARBONYL COMPOUNDSBOOK - FULL MARKSCHAPTER - CARBONYL COMPOUNDSEXERCISE - ADDITIONAL QUESTIONS (2 MARKS QUESTIONS)
67 Videos
- CARBONYL COMPOUNDSBOOK - FULL MARKSCHAPTER - CARBONYL COMPOUNDSEXERCISE - ADDITIONAL QUESTIONS (MATCH THE FOLLOWING)
12 Videos
- BIOMOLECULES BOOK - FULL MARKSCHAPTER - BIOMOLECULES EXERCISE - ADDITIONAL QUESTIONS (5-MARK QUESTIONS)
12 Videos
- CHEMICAL KINETICSBOOK - FULL MARKSCHAPTER - CHEMICAL KINETICSEXERCISE - ADDITIONAL QUESTIONS (5 MARK QUESTIONS)
10 Videos
Similar Questions
Find the odd one out and give the reasons.
View SolutionWrite the name of the following compounds.
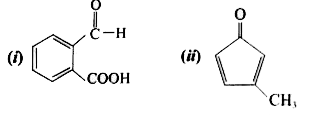
View SolutionMatch the column I and II using the code given below the column

View SolutionHow would you prove the strutcure of glucoe ? (OR) Elucidate the strcture of glucose .
View SolutionHow would you calculate the order of the reaction 2NO(g)+O2(g)→2NO2(g) by an experiment ? (or) prove that 2NO+O2→2NO2 is a third order reaction.
View SolutionCarboxylic acid have higher boiling points than aldehydes, ketones and even alcohol of comparable molecular mass. It is due to their
View Solutionकार्बोक्सिलिक अम्लों के क्वथनांक तुलनीय अणुभार युक्त ऐल्डिहाइड्स, कीटोन्स और ऐल्कोहॉल्स से भी अधिक होते हैं। इसका मुख्य कारण है-
View SolutionCarboxylic acids have higher boiling points than aldehydes, ketones and even alcohols of comparable molecular mass. It is due to their
View Solutionलगभग समान अणुभार वाले ऐल्डिहाइड , कीटोन व ऐल्कोहॉल की तुलना में कार्बोक्सिलिक अम्लों का क्वथनांक अधिक होने का कारण है-
View SolutionCarboxylic acids have higher boiling points than aldehydes, ketones and even alcohols of comparable molecular mass. It is due to their-
View Solutionऐल्डिहाइड /कीटोन के क्वथनांक कार्बोक्सिलिक अम्लों से कम होते है। क्यों ?
View SolutionCarboxylic acids have higher boiling points than aldehydes, ketones and even alcohols of comparable molecular mass. It is due to their
View SolutionAssertion(A): Carboxylic acids have higher boiling point than aldehyde and ketone of comparable molecular mass. Reason (R): This is due to more association of carboxylic acid. Molecules through intermolecular hydrogen bonding.
View SolutionCarboxylic acids have higher boiling point than aldehyde, keton and even alcohols of comparable molecular masses. Give reason.
View SolutionThe boiling points of carboxylic acids have higher molecular mass than aldehydes, ketones, and even alcohols. What causes it
View Solution

