What is Clemmensen reduction ? Explain it.
Text Solution
Aldehyde and ketones when heated with zinc amalgam and concentrated hydrochloric acid give hydrocarbons. This reaction is known as Clemmensen reduction.
CH3−C∣∣O−H+4[H]acetaldehydeZn+Hg−−−−−−−→conc.HClCH3−CH3+H2Oethane
|
Similar Questions
Knowledge Check
The reagent used in Clemmensen's reduction is
Aconc. H2SO4BZn-Hg/conc. HClCaq. KOHDalc.KOHThe Clemmensen reduction of acetone yields
AEthanolBEthanalCPropaneDPropanol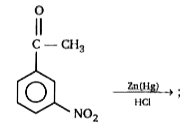 , Product of the Clemmensen reduction is :A
, Product of the Clemmensen reduction is :A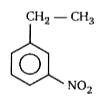 B
B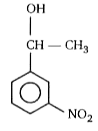 C
C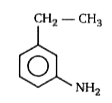 D
D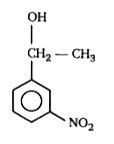
Write the reactions involved in the following reactions:
(i) Clemmensen reduction
(ii) Cannizzaro reaction
View SolutionWrite the reactions involved in the following reactions:
(i) Clemmensen reduction
(ii) Cannizzaro reaction
View SolutionWrite a note on Clemmensen reduction.
View Solution
Product of the Clemmensen reduction is :
View Solution
Product of the Clemmensen reduction is ,
View Solution

