Aldehydes and ketones have high boiling point as compared to hydrocarbons and ethers of comparable molecular mass and less than that of alcohols. Give reason.
Aldehydes and ketones have high boiling point as compared to hydrocarbons and ethers of comparable molecular mass. It is due to the weak molecular association in aldehyde and ketone arising out of the dipole-dipole interactions .
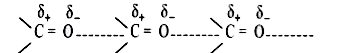
These dipole-dipole interactions are weaker than intermolecular H-bonding. The boiling points of aldehydes and ketones are much lower than those of corresponding alcohols and carboxylic acids which possess inter molecular hydrogen bonding.
Similar Questions
Carboxylic acids have higher boiling point than aldehyde, keton and even alcohols of comparable molecular masses. Give reason.
View SolutionCarboxylic acids have higher boiling points than aldehydes, ketones and even alcohols of comparable molecular mass. It is due to their
View SolutionExplain why the boiling points of aldehyde and ketone are higher than hydrocarbons and ethers of comparable molecular masses?
View SolutionCarboxylic acid have higher boiling points than aldehydes, ketones and even alcohol of comparable molecular mass. It is due to their
View SolutionCarboxylic acids have higher boiling points than aldehydes, ketones and even alcohols of comparable molecular mass. It is due to their
View SolutionAssertion: Alcohols have higher boiling points than ethers of comparable molecular masses.
Reason: Alcohols and ethers are isomeric in nature.
View SolutionAssertion: Alcohols have higher boiling points than ethers of comparable molecular masses.
Reason: Alcohols and ethers are isomerism in nature.
View Solution

