Which one of the following is a batter nucleophile and why?
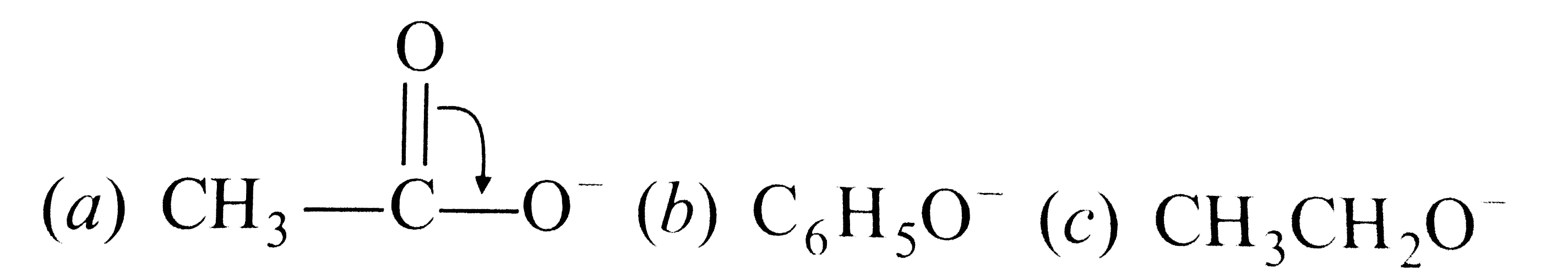
Video Solution
Text Solution
CH3CH2O− is a good nucleophile becauce the −ve charge is concentrated on one oxgyen only and the rate is directly proportional to the basicity.
(a) CH3COO− is a resonance hybrid of the following structures and the −ve charge is divided on the two carbon atoms.
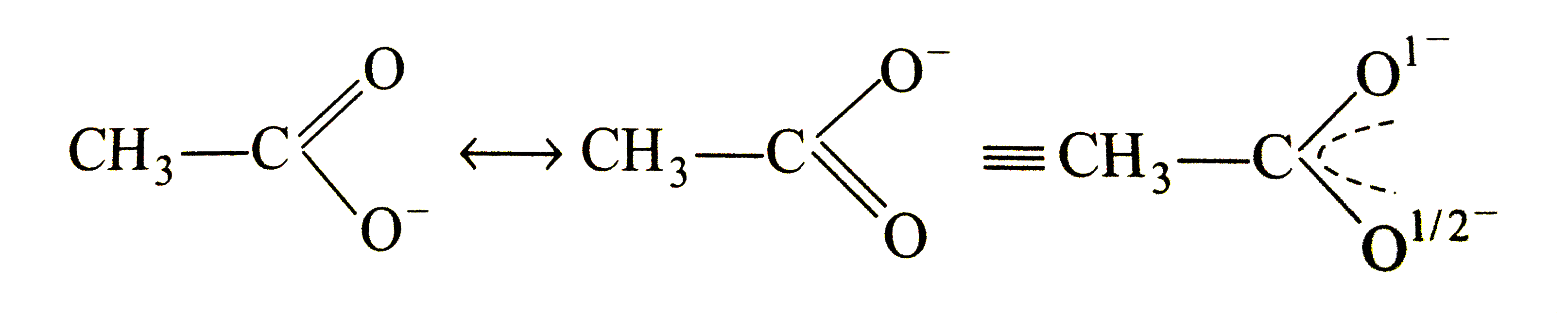
(b) C6H5O− is not a good nucleophile because the −ve charge is distributed at four positions.
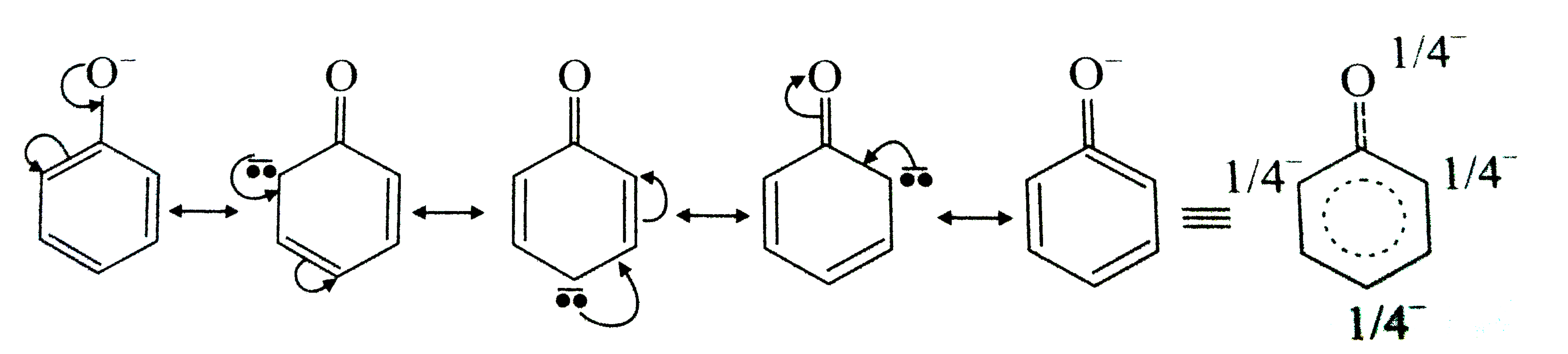
|
Similar Questions
Knowledge Check
Which of the following is ambidantate nucleophile?
AOHBNH2CRODCNWhich of the following is an ambident nucleophile ?
AH+BCN−CCl+DOH−Which of the following is an ambident nucleophile :
AHSOΘ3BCNΘCNO2ΘDAll of these

