The SN1 reactivity of the following halides will be in the order:
(i) (CH3)3C−Br
(ii) (C6H5)CHBr
(iii) (C6H5)2C(CH3)Br
(iv) (CH3)2CHBr
(v) C2H5Br
(v) gt (iv) gt (iv) gt (iii)
(ii) gt (i) gt (iii) gt (v) gt (iv)
(i) gt (iii) gt (v) gt (ii) gt (iv)
(iii) gt (ii) gt (i) gt (iv) gt (v)
The correct Answer is:D
Step by step video, text & image solution for The S_(N^(1)) reactivity of the following halides will be in the order: (i) (CH_(3))_(3)C-Br (ii) (C_(6)H_(5))CHBr (iii) (C_(6)H_(5))_(2)C(CH_(3))Br (iv) (CH_(3))_(2)CHBr (v) C_(2)H_(5)Br by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Classify the following as alkyl, vinyl, allyl or aryl halides :
(i) H2C=CHCHI2 (ii) CH3CH=CFCH2CH3 (iii)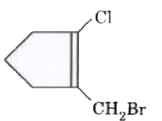
(iv) (CH3)2CClCH2CH3 (v) (CH3)2C=CHCH2Br (vi) C6H5Br
View SolutionClassify the following as alkyl, vinyl, allyl or aryl halides :
(i) H2C=CHCHI2 (ii) CH3CH=CFCH2CH3 (iii)
(iv) (CH3)2CClCH2CH3 (v) (CH3)2C=CHCH2Br (vi) C6H5Br
View Solution
Knowledge Check
SN1 reactivity of the following halides will be in the order
> (i) (CH3)3Br (ii) (C6H5)2CHBr
> (iii) (C6H5)2C(CH3)Br (iv) (CH3)2CHBr
> (v) C2H5BrA(v) > (iv) > (i) > (ii) > (iii)B(ii) > (i) > (iii) > (v) > (iv)C(i) > (iii) > (v) > (ii) > (iv)D(iii) > (ii) > (i) > (iv) > (v)The order of reactivity of following alkyl halides towards SN1 reaction is
(i)(CH3)3CBr , (ii)(C6H5)2CHBr, (iii)(C6H5)2C(CH3)Br , (iv)(CH3)2CHBr , (v)C2H5BrA(v)>(iv)>(i)>(ii)>(iii)B(ii)>(i)>(iii)>(v)>(iv)C(i)>(iii)>(v)>(ii)>(iv)D(iii)>(ii)>(i)>(iv)>(v)The decreasing order of reactivity of the following compounds in SN1 reactions is _________.
(i) C6H5CH2Br (ii)C6H5CH(C6H5Br
(iii)C6H5CH(CH3Br (iv)C6H5C(CH3)(C6H5)BrA(i)>(ii)>(iii)>(iv)B(iv)>(iii)>(ii)>(i)C(iv)>(ii)>(iii)>(i)D(iii)>(ii)>(i)>(iv)
(a) Indicate whether the following are SN1,SN2,E1 or E2 reactions.
(i)CH3CH2CH2CI+I−→
(ii)(CH3)3CBr+CN−(alc.)→
(iii)CH3CH(Br)CH3+OH−(alc.)→
(iv)(CH3)3C−Br+H2O→
(b) Give the decreasing order of reactivity of the alkaline hydrolysis of the following alkyl halides toward (A)SN1(B)SN2(C) in pressure of AgNO3.
(i)(CH3)2C(Br)−C2H5(ii)C5H11Br
(iii)C2H5CH(Br)C2H5
(c) Give the decreasing order reactivity of the alkaline hydrolysis of hte following halides towards SN1(i)MeCI(ii)PhCH2CI(ii)Ph2CHCI(iii)Ph3C−CI.
(d) Determine the relative SN2 reactivity with NaCI in aprotic DMSO of the following alkyl halides :
(A)(i)CH3CHCICH2CH3(ii)CH3CHBrCH2CH3
(iii)CH3CHICH2CH3
(B)(i)CH3CH(CH3)CH2CH2I
(ii)CH3CH(CH3)CHICH3(iii)CH3CI(CH3)CH2CH3
(e) Arrange the following in the order of decreasing nucleophilic character:
H2O,−OH,CH3O−,CH3COO−
(f) Arrange the following in the decreasing order as leaving group in SN reaction:
CH3COO−,C6H5O−,C6H5SO−3,CH3O−
View SolutionThe number of reactions proceeding through free radical mechanism are
(i) C2H5Br+Na+−OCH3
(ii) C2H5Br+AgCN
(iii) C6H5CH3+Cl2(hv)
(iv) CH3CH=CH2+HBr (peroxides)
(v) C2H5Br+alc.KOH
(vi) CH3CH=CH2+HCl(peroxide)
(vii) C2H5Br+H2(NiorPd)
(viii) C2H6+Cl2(hv)
(ix) C2H5Br+NaSH
View SolutionThe order of decreasing stability of the following carbanions is
(i) (CH3)3C−
(ii) (CH3)2CH−
(iii) CH3CH−2
(iv) C6H5CH−2
View SolutionMost stable carbocation formed from (CH3)3C−Br,(C6H5)3CBr,(C6H5)2CHBr and C6H5CH2Br would be
View SolutionThe order of reactivity of the following compounds towards electrophilic substitution will be :
(I) C6H5CH3
(II) C6H5CH2CH3
(III) (C6H5CH(CH3)3
(IV) (C6H5C(CH3)3
View Solution
