An aqueous solution of CuSO4 is subjected to electrolysis using inert electrodes.The pH of the solution will Solution in Kannada
increase
remains unchanged
decrease
increase or decrease depending on the strength of the current.
The correct Answer is:C
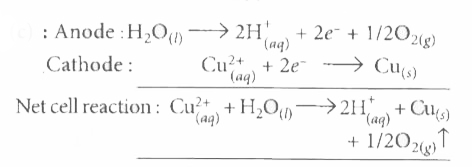
Due to increase in [H+], pH decreases .
Similar Questions
Aqueous solution of AgNO3 is electrolysed using inert electrodes. At the end of electrolysis
View SolutionPredict the products of electrolysis in each of the following
i) An aqueous solution of CuSO4 with Copper electrodes
ii) An aqueous solution CuSO4 with plantinum electrodes
View SolutionIf electrolysis of aqueous solution of CuSO4 is carried out using graphite electrode, then what is the pH of solution of electrolytic cell ?
View SolutionDuring electrolysis of dilute aqueous CuSO4 solution by inert electrode, the pH of solution is . . .
View SolutionAqueous solution of CuSO4 is electrolysed using inert electrodes till the blue coloured solution becomes colourless. The colourless solution formed is
View SolutionAqueous solution of AgNO3 is electrolysed using inert electrodes. At the end of electrolysis
View SolutionAn aqueous solution of CuSO4 is subjected to electrolysis using inert electrodes.The pH of the solution will
View SolutionWhat will be PH of aqueous solution of electrolyte in electrolytic cell during electrolysis of CuSO4(Aq) between graphite electrodes ?
View Solution
