Orthophosphoric acid (H3PO4) is not a reducing agent wheres hypophosphorus acid (H3PO2) is a strong reducing agent "Explain and justify the above statement with the help of a suitable example.
Video Solution
Text Solution
The structure of H3PO2 has two P-H bonds. Due to the presence of these P-H bonds, H3PO2 act as a strong reducing agent.
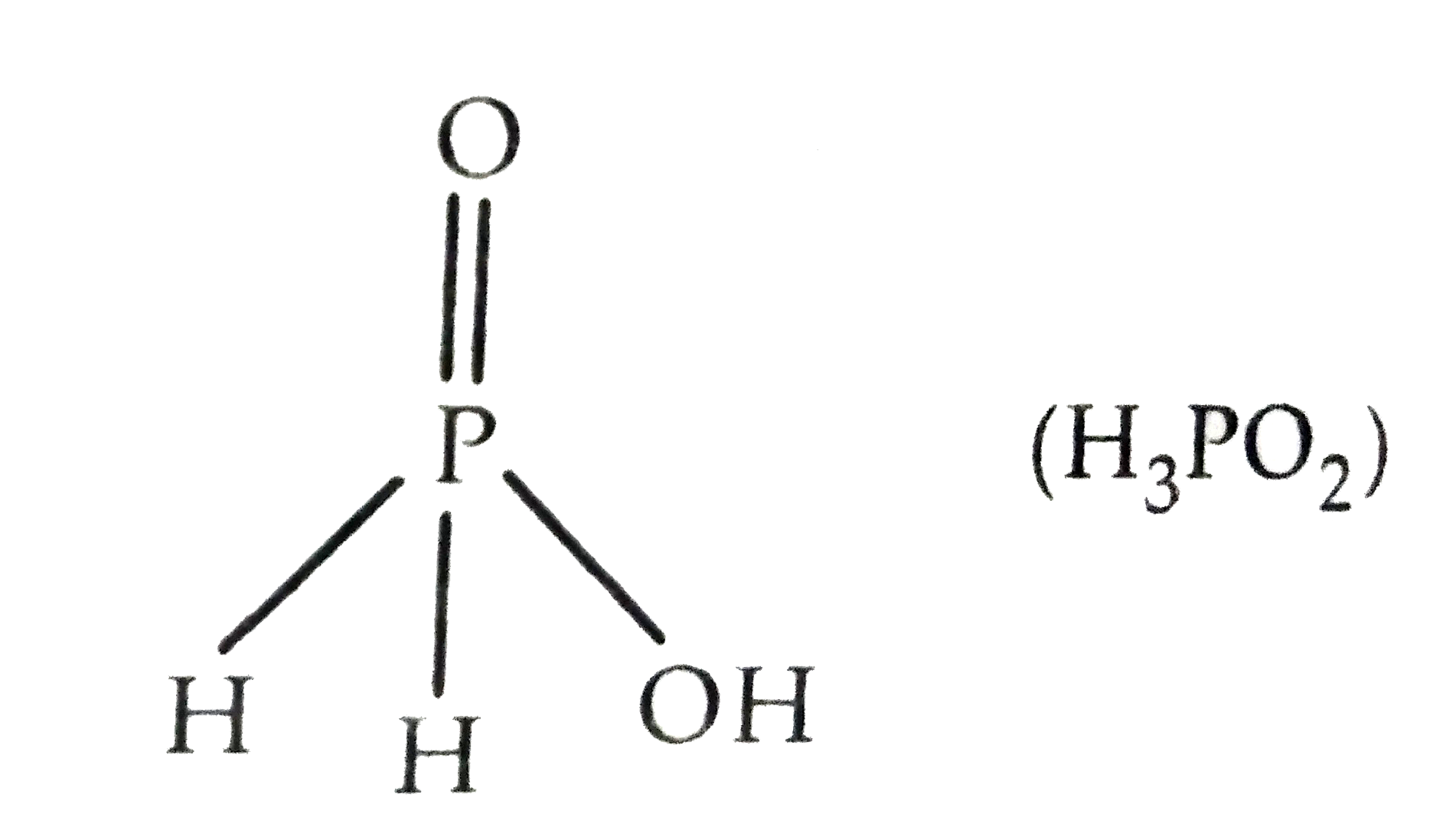
Example 4AgNO3+H3PO2+2H2O→4Ag+H3PO4+4HNO3
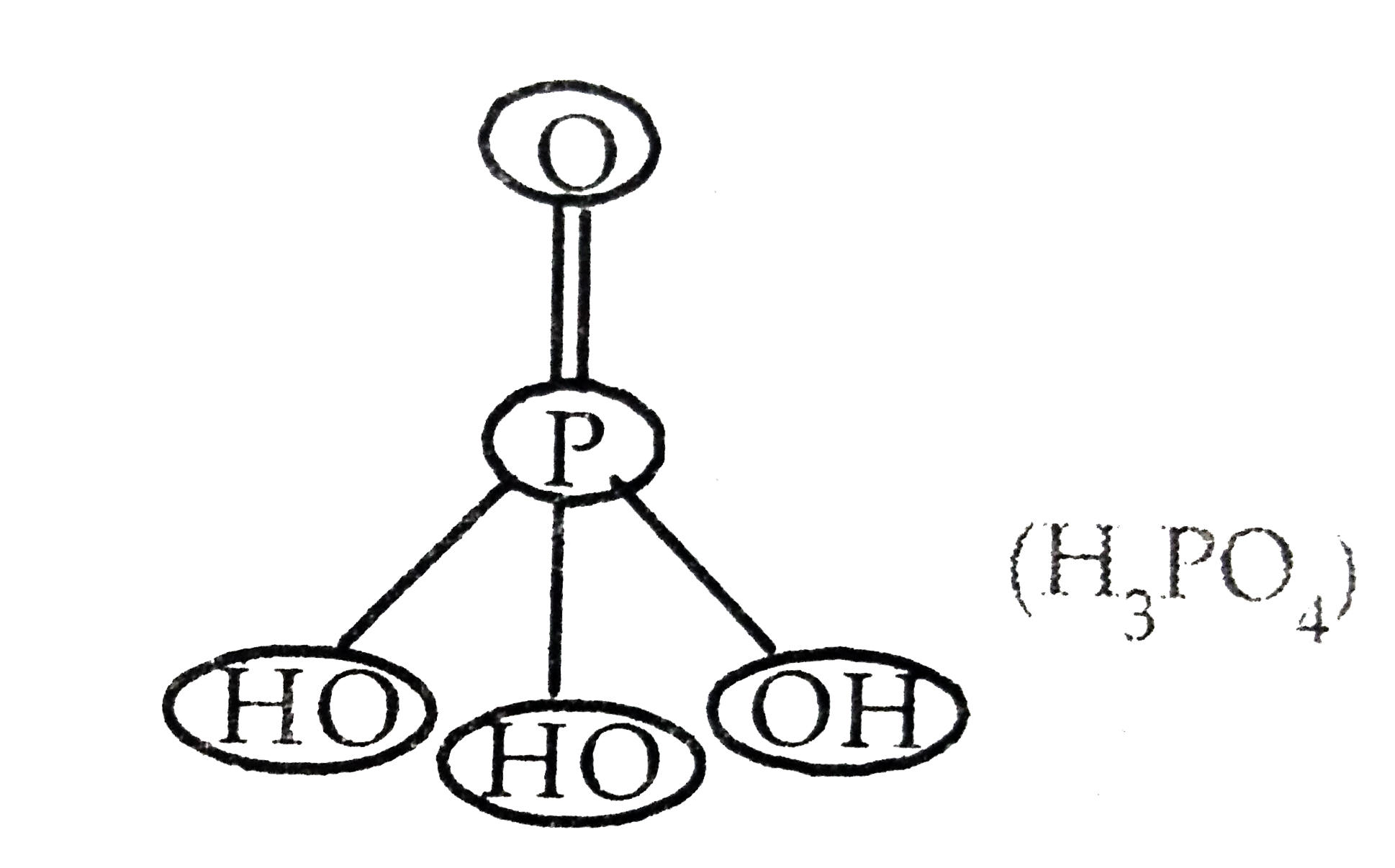
Whereas H3PO4 has all P−OH. Hence due to the absence of P-H bonds, it is not a reducing agent.
|
Similar Questions
Knowledge Check
The basicity of phosphorus acid (H3PO3) is ............ .
AoneBtwoCfourDthreePhosphoric acid H3PO4 can not be neutralised to :
AHPO2−4BPO2−4CH2PO−4DHPO2−3H3PO4 is an example of
Amonobasic acidBdibasic acidCtribasic acidDstrong acid

