(a) Why phenol is more acidic than than ethanol ?
(b) Write the mechanism of acid dhydration of ethanol to tield ether:
2CH3CH2OHH+−−→413KCH3CH2OCH2CH3
(a) 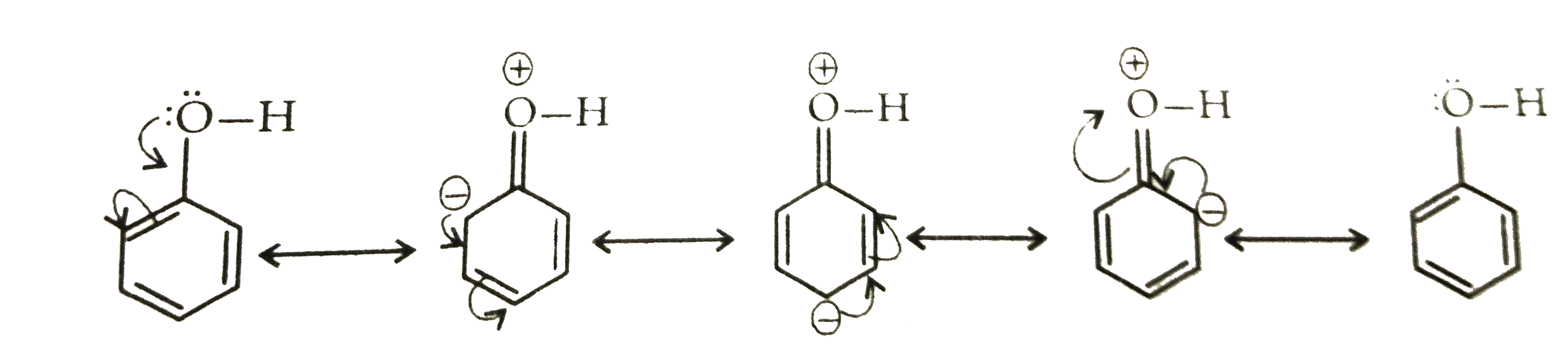
Due to resonance, the oxygen atom acquires a partial positive charge. This weaknes the O-H bond an thus facilitate the release of a proton. Conversely, +I effect of ethyl group increase electron density which make O-H bond cleavage difficullt. Hence ethanol less acidic than phenol.
(b) 2CH3CH2OHH+−−→413KCH3CH2OCH2CH3
Mechanism:
(i) Protonation
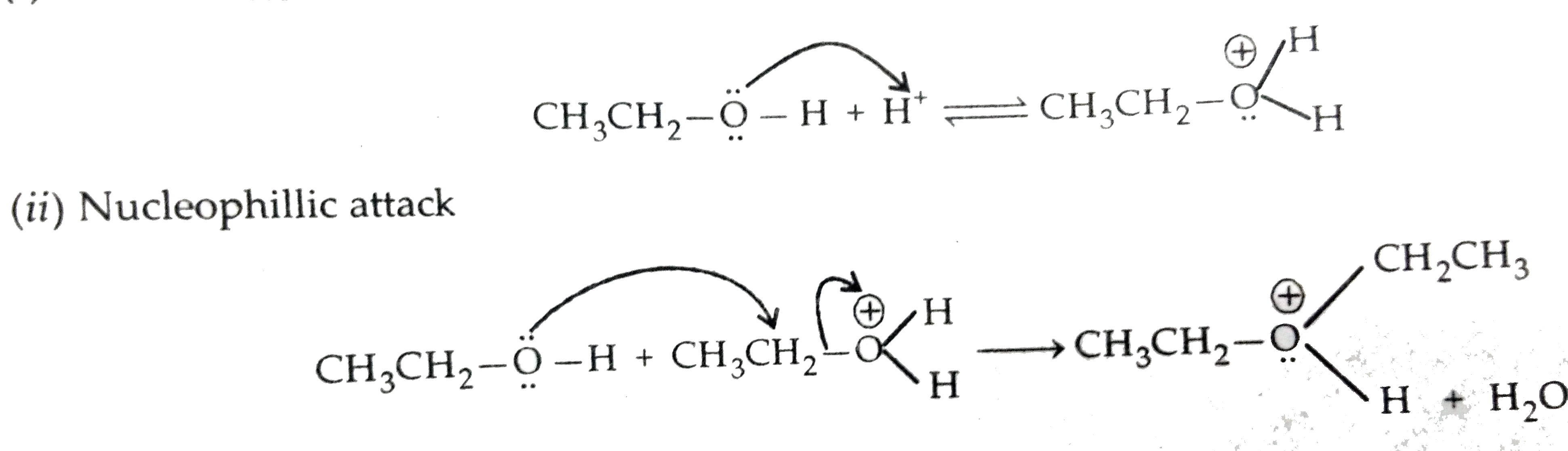
(ii) Nucleophilic attack
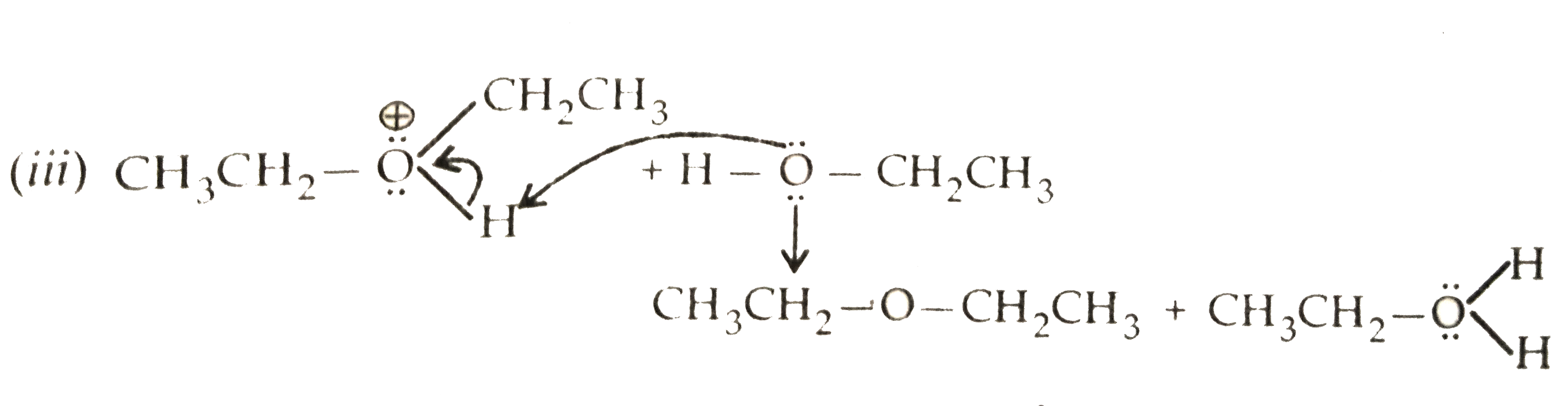
Similar Questions
Write the mechanism of the following reaction :
2CH3CH2OHConc. H2So4−−−−−−−→413KCH3CH2−O−CH2−CH3
View SolutionWrite the mechanism of the following reaction :
2CH3CH2OHConc. H2So4−−−−−−−→413KCH3CH2−O−CH2−CH3
View SolutionCH3OCH2CH2OH∣CHCH2Cl
View SolutionExplain the mechanism of the reaction is given below :
2CH3−CH2−OHH+−−→413KCH3CH2−O−CH2−CH3+H2O
View SolutionExplain the mechanism of the reaction is given below :
2CH3−CH2−OHH+−−→413KCH3CH2−O−CH2−CH3+H2O
View SolutionWrite the mechanism of the following reaction :
2CH3CH2OhConc. H2SO4−−−−−−−−→413KCH3CH2−O−CH2−CH3
View SolutionWrite mechanism of the following reaction:
CH3CH2OHHBr−−→CH3CH2Br+H2O
View SolutionWrite mechanism of the following reaction:
CH3CH2OhHBr−−→CH3CH2Br+H2O.
View Solution(a) How do you convert the following:
(i) Phenol to Anisole (ii) Ethanol to Propan-2-ol
(b) Write mechanism of the following reaction:
C2H5OhH2SO4−−−→443KCH2=CH2+H2O
(c) Why phenol undergoes electrophilic substitution more easily than benzene?
View Solution(a) How do you convert the following:
(i) Phenol to Anisole (ii) Ethanol to Propan-2-ol
(b) Write mechanism of the following reaction:
C2H5OhH2SO4−−−→443KCH2=CH2+H2O
(c) Why phenol undergoes electrophilic substitution more easily than benzene?
View Solution

